New insights into interstitial cystitis/bladder pain syndrome at single-cell resolution
Abstract
Objective
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic inflammatory bladder disorder with unknown aetiology and limited treatment options. Single-cell RNA-sequencing (scRNA-seq) has provided unprecedented insights into cellular heterogeneity in IC/BPS. This review summarizes recent scRNA-seq findings on bladder cell populations, emphasizing urothelial, interstitial and immune cells.
Methods
A comprehensive analysis of published scRNA-seq studies was conducted to compare bladder cell subtypes in healthy and IC/BPS-affected bladders. Differences between IC/BPS patients and mouse models, as well as sex-specific cellular variations, were examined.
Results
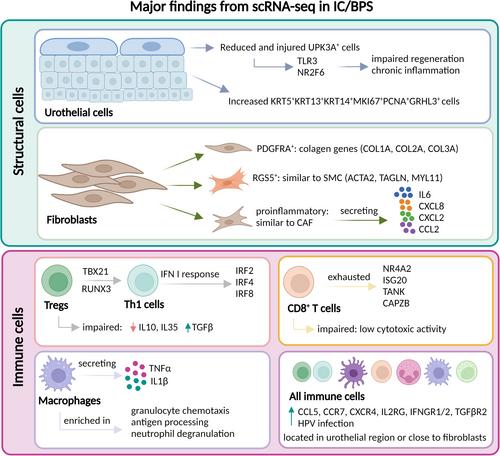
IC/BPS bladders exhibit significant urothelial alterations, including a reduction in UPK3A + umbrella cells and an expansion of progenitor-like cells with impaired regenerative capacity, linked to TLR3-NR2F6 signalling. Interstitial cells include three fibroblast subtypes (PDGFRA+, RGS5+ and pro-inflammatory IL6-producing fibroblasts), which contribute to fibrosis and inflammation. The immune landscape is characterized by a Th1-biased response, exhausted CD8 + T cells and reduced regulatory T cells, with HPV infection detected in most IC/BPS patients, suggesting a possible viral aetiology. Cell-to-cell interactions are compromised, with enhanced macrophage-endothelial signalling via CXCL8-ACKR1 and CXCL2/3-ACKR1 pathways, highlighting potential therapeutic targets. Notably, sex-based differences reveal stronger immune activation in females and increased urothelial proliferation in males, potentially explaining the higher IC/BPS prevalence in females.
Conclusions
scRNA-seq has advanced our understanding of IC/BPS by identifying disease-associated cell types, signalling pathways and intercellular interactions. Future research should integrate multi-omics approaches and explore non-invasive urine-based scRNA-seq for improved diagnosis and therapy.



 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


