Total Synthesis of (−)-Steganacin via Stereocontrolled Atroposelective Intramolecular Mizoroki-Heck Reaction and Evaluation of Biological Activity
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
(−)-Steganacin is a lignan with a unique dibenzocyclooctadiene lactone framework. Herein, we describe the total synthesis of (−)-steganacin and its congeners through a stereocontrolled atroposelective intramolecular Mizoroki-Heck reaction. The synthesis of (−)-steganacin was completed in 11 steps from commercial substrates with a 7% overall yield. Furthermore, the biological activities of (−)-steganacin and its synthetic analogues were evaluated. (−)-Steganacin exhibits potent antiproliferation activity against MDA-MB-468 breast cancer cells with IC50 = 0.50 μM.
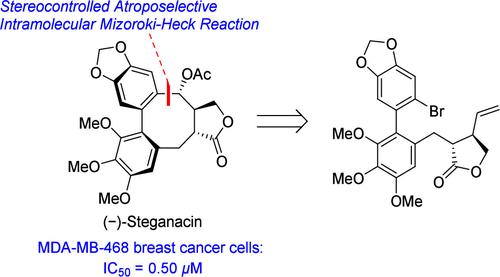
立体控制atroo选择性分子内Mizoroki-Heck反应合成(-)-司地那酸及其生物活性评价。
(-)- steganacin是一种木脂素,具有独特的二苯并环二烯内酯结构。在这里,我们描述了通过一个立体控制的atroopselective分子内Mizoroki-Heck反应的(-)-steganacin及其同族物的全合成。从商业底物合成(-)-斯特甘纳星共11步,总收率为7%。此外,还对(-)-斯特甘纳星及其合成类似物的生物活性进行了评价。(-)- steganacin对MDA-MB-468乳腺癌细胞具有较强的抗增殖活性,IC50 = 0.50 μM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


