OseR, a bacterial redox sensor, regulates ergothioneine uptake via a Cys thiol switch, enhancing oxidative stress resistance and virulence
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
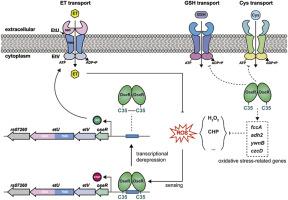
Ergothioneine (ET), a low-molecular-weight (LMW) thiol, serves as a potent antioxidant. While only a limited number of Actinomycetes and fungi can synthesize ET, most microorganisms acquire it from external sources. Recently, a microbial ET transporter system (EtUV) was identified in Helicobacter pylori and Streptococcus pneumoniae, but the regulatory mechanisms controlling EtUV in bacteria remain unknown. In this study, we identified and characterized OseR, a novel MarR family repressor in Streptococcus suis, a significant pathogen causing systemic diseases such as septicemia and meningitis in pigs and humans. We demonstrated that OseR senses oxidative stress through a thiol switch at Cys35, which regulates the ET transport system EtUV. Under oxidative stress, OseR dissociates from the promoter region of the ET transport operon due to the formation of an intermolecular disulfide bond, leading to the activation of EtUV expression. Our findings reveal that OseR not only controls ET transport but also modulates other LMW thiol transport pathways, including glutathione and cysteine, as well as genes involved in oxidative stress responses. Deletion or mutation of oseR significantly impairs oxidative stress tolerance, survival in mouse macrophages, and virulence in mice. Similarly, deletion or mutation of etU, which encodes a transmembrane permease essential for ET uptake, markedly reduces oxidative stress tolerance and virulence in mice. Importantly, our results suggest that OseR-mediated regulation of the ET transport system, driven by a thiol-based switch, may be conserved across bacterial species, highlighting a broader role for OseR in bacterial adaptation to host environments. This study advances our understanding of the regulatory mechanisms governing ET uptake in bacteria and provides new insights into the link between ET and bacterial pathogenicity.
OseR是一种细菌氧化还原传感器,通过Cys硫醇开关调节麦角硫因的摄取,增强氧化应激抗性和毒力。
麦角硫因(ET)是一种低分子量(LMW)硫醇,是一种有效的抗氧化剂。虽然只有有限数量的放线菌和真菌可以合成ET,但大多数微生物从外部来源获得ET。最近,在幽门螺杆菌和肺炎链球菌中发现了微生物ET转运体系统(EtUV),但细菌中控制EtUV的调控机制尚不清楚。在这项研究中,我们鉴定并鉴定了猪链球菌中一种新的MarR家族抑制因子OseR,猪链球菌是一种引起猪和人败血症和脑膜炎等系统性疾病的重要病原体。我们证明OseR通过Cys35的巯基开关感知氧化应激,该开关调节ET转运系统EtUV。氧化应激下,OseR与ET转运操纵子启动子区分离,形成分子间二硫键,激活EtUV表达。我们的研究结果表明,OseR不仅控制ET转运,还调节其他LMW硫醇转运途径,包括谷胱甘肽和半胱氨酸,以及参与氧化应激反应的基因。oseR的缺失或突变显著损害小鼠氧化应激耐受性、巨噬细胞的存活和小鼠的毒力。同样,etU的缺失或突变会显著降低小鼠的氧化应激耐受性和毒力。etU编码一种对ET摄取至关重要的跨膜渗透酶。重要的是,我们的研究结果表明,由巯基开关驱动的OseR介导的ET转运系统调节可能在细菌物种中是保守的,这突出了OseR在细菌适应宿主环境中的更广泛作用。这项研究促进了我们对细菌摄取ET的调节机制的理解,并为ET与细菌致病性之间的联系提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


