Pancreatic stellate cells have adipogenic and fibrogenic potentials but only show increased pro-fibrogenic propensity upon aging
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Steatosis and fibrosis are two key pathological features of the aging pancreas that contribute to inflammation, metabolic dysfunction and degenerative changes of the tissue. Pancreatic stellate cells (SCs) are thought to be the main cellular source of ectopic adipocytes and fibrotic structures. SCs are fibroblast-like mesenchymal cells that reside in various microanatomies of the tissue and whose task under healthy, homeostatic conditions is to generate extracellular matrix (ECM) and maintenance signals to ensure tissue integrity and function. To examine pathological changes of the aging pancreas, we conducted single cell RNA-sequencing to analyze murine pancreatic cell heterogeneity and examined morphological changes of the aging exocrine pancreas, comparing young adult and aged wildtype mice. We specifically focused our analyses on SCs and their cellular interaction partners and mechanisms of paracrine crosstalk. Age-dependent transcriptional differences in these cell types occur on the level of ECM-related and pro-fibrotic expression signatures mediated through transforming growth factor (TGF) and platelet-derived growth factor (PDGF) signaling pathways. SCs can be divided into distinct subsets of activated (aSCs) and quiescent stellate cells (qSCs), based on distinct expression signatures and transcript levels of Pdgfra and Pdgfrb, which encode for the PDGF receptor alpha and -beta paralogs. Activated SCs, which display PDGF receptor alpha (PDGFRα) on their surface, exhibited subsets that appeared to be either pro-adipogenic or pro-fibrogenic at the transcriptomic level and aging promoted a pro-fibrogenic switch in aSCs. We conclude that pancreatic SCs are contributors to the age-related decline of the exocrine pancreas through an increased contribution of a pro-fibrotic microenvironment.
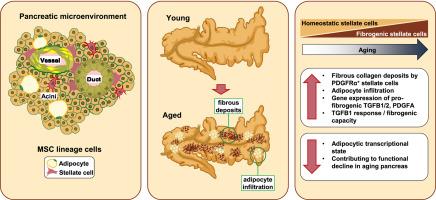
胰腺星状细胞具有成脂肪和成纤维的潜力,但随着年龄的增长只表现出促成纤维的倾向。
脂肪变性和纤维化是衰老胰腺的两个关键病理特征,可导致炎症、代谢功能障碍和组织退行性变化。胰腺星状细胞(SCs)被认为是异位脂肪细胞和纤维化结构的主要细胞来源。SCs是一种类似成纤维细胞的间充质细胞,存在于组织的各种微观解剖结构中,在健康、稳态条件下,其任务是产生细胞外基质(ECM)和维持信号,以确保组织的完整性和功能。为了研究衰老胰腺的病理变化,我们通过单细胞rna测序分析小鼠胰腺细胞的异质性,并比较年轻成年小鼠和老年野生型小鼠衰老外分泌胰腺的形态学变化。我们特别关注SCs及其细胞相互作用伙伴和旁分泌串扰机制的分析。在这些细胞类型中,年龄依赖性的转录差异发生在通过转化生长因子(TGF)和血小板衍生生长因子(PDGF)信号通路介导的ecm相关和促纤维化表达信号水平上。基于编码PDGF受体α和- β的Pdgfra和Pdgfrb不同的表达特征和转录水平,SCs可以分为不同的激活(aSCs)和静止星状细胞(qSCs)亚群。活化的SCs表面显示PDGF受体α (PDGFRα),在转录组水平上显示出促脂肪生成或促纤维生成的亚群,衰老促进了aSCs中促纤维生成的开关。我们得出结论,胰腺SCs通过促纤维化微环境的增加,促进了外分泌胰腺的年龄相关性衰退。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


