Structural insights of the complex formed by KRAS G12V and a novel TIG3 peptide
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2025-07-31
DOI:10.1016/j.bbapap.2025.141091
引用次数: 0
Abstract
Pancreatic cancer remains a severe malignancy with a dismal 5-year survival rate, and most pancreatic cancer patients harbor KRAS mutations, which are critical targets for anti-cancer drug development. However, the structural characteristics of KRAS present challenges for therapeutic targeting. In this study, we developed a novel peptide derived from TIG3 protein, a type II tumor suppressor, and confirmed its moderate affinity binding to KRAS G12V. X-ray crystallography revealed that this peptide binds near the Switch II domain of KRAS G12V, causing conformational changes likely to affect its activity. Furthermore, the peptide reduced the viability of cancer cell lines harboring the KRAS G12V mutation, thus demonstrating its potential as a KRAS G12V inhibitor. Our results indicate that the developed novel TIG3 peptide is a promising candidate for KRAS-targeted therapy and provide structural insights useful for the development of pancreatic ductal adenocarcinoma therapeutics.
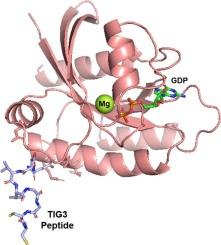
KRAS G12V和一种新的TIG3肽形成复合物的结构见解。
胰腺癌仍然是一种严重的恶性肿瘤,其5年生存率很低,大多数胰腺癌患者携带KRAS突变,这是抗癌药物开发的关键靶点。然而,KRAS的结构特点给靶向治疗带来了挑战。在本研究中,我们从II型肿瘤抑制因子TIG3蛋白中提取了一种新的肽,并证实其与KRAS G12V具有中等亲和力结合。x射线晶体学显示,该肽结合在KRAS G12V的Switch II结构域附近,引起构象变化,可能影响其活性。此外,该肽降低了携带KRAS G12V突变的癌细胞系的生存能力,从而证明了其作为KRAS G12V抑制剂的潜力。我们的研究结果表明,开发的新型TIG3肽是kras靶向治疗的有希望的候选者,并为胰腺导管腺癌治疗方法的发展提供了有用的结构见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


