Insights into early cochlear damage induced by potassium channel deficiency
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-08-05
DOI:10.1016/j.bbamcr.2025.120030
引用次数: 0
Abstract
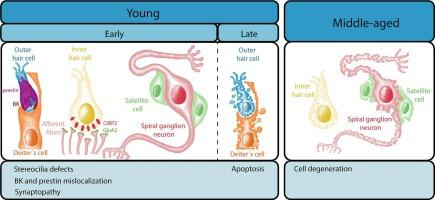
Hearing loss (HL) is the most common sensory disorder, caused by genetic mutations and acquired factors like presbycusis and noise exposure. A critical factor in HL development is the dysfunction of potassium (K+) channels, essential for sensory cell function in the organ of Corti (OC). Inner and outer hair cells (IHCs and OHCs) convert sound into electrical signals, while supporting cells (SCs) maintain ionic and structural balance. KCNQ4 channels, located in the basal membrane of OHCs, regulate K+ efflux. Mutations in KCNQ4 are linked to progressive HL (DFNA2), noise-induced hearing loss, and presbycusis, leading to K+ accumulation, cellular stress, and OHC death. Gene editing or pharmacological activation of KCNQ4 has shown potential in partially preventing HL in mouse models. In this study, we demonstrate KCNQ4 deletion disrupts the localization of key proteins like prestin and BK channels, alters OHC organization, and induces apoptosis in sensory and SC. Spiral ganglion neurons (SGNs) also degenerate over time. Despite these structural changes, noise exposure does not exacerbate OHC damage in our KCNQ4-deficient model. This highlights KCNQ4's role in maintaining ion homeostasis and cochlear function, as its absence triggers widespread dysfunction in the OC. The present study demonstrates that disruptions in a single cell type can have a cascade effect on overall cochlear health. Understanding the molecular and cellular consequences of KCNQ4 mutations is crucial for developing targeted therapies to mitigate progressive HL caused by genetic and environmental factors.
钾通道缺乏致早期耳蜗损伤的研究进展。
听力损失(HL)是最常见的感觉障碍,由基因突变和获得性因素如老年性耳聋和噪音暴露引起。HL发展的一个关键因素是钾(K+)通道的功能障碍,这对Corti器官(OC)的感觉细胞功能至关重要。内外部毛细胞(IHCs和OHCs)将声音转化为电信号,而支持细胞(SCs)维持离子和结构平衡。KCNQ4通道位于OHCs基膜,调节K+外排。KCNQ4突变与进行性HL (DFNA2)、噪声性听力损失和老年性耳聋有关,导致K+积累、细胞应激和OHC死亡。在小鼠模型中,基因编辑或药理激活KCNQ4已显示出部分预防HL的潜力。在这项研究中,我们证明KCNQ4缺失会破坏prestin和BK通道等关键蛋白的定位,改变OHC组织,诱导感觉和SC的凋亡。螺旋神经节神经元(sgn)也会随着时间的推移而退化。尽管存在这些结构变化,但在kcnq4缺陷模型中,噪声暴露不会加剧OHC损伤。这突出了KCNQ4在维持离子稳态和耳蜗功能中的作用,因为它的缺失会引发耳蜗OC中广泛的功能障碍。目前的研究表明,单个细胞类型的破坏可以对整个耳蜗健康产生级联效应。了解KCNQ4突变的分子和细胞后果对于开发靶向治疗以减轻由遗传和环境因素引起的进行性HL至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


