An RMST-based analysis of systemic therapy in muscle-invasive bladder cancer
IF 2.3
3区 医学
Q3 ONCOLOGY
Urologic Oncology-seminars and Original Investigations
Pub Date : 2025-08-05
DOI:10.1016/j.urolonc.2025.07.002
引用次数: 0
Abstract
Introduction
Recent Phase III trials using immunotherapy agents in perioperative setting for muscle-invasive bladder cancer (MIBC) have reshaped treatment paradigms. Traditional hazard ratios, while statistically robust, may mask modest absolute survival gains, complicating informed decision-making for clinicians and patients alike. This study aims to utilize restricted mean survival time (RMST) analysis to provide more intuitive interpretations of survival benefits and facilitate development of a framework for comparing efficacy, toxicity, and costs across three pivotal trials.
Methods
Overall survival (OS), disease free survival (DFS) and adverse effects (AE) data were extracted from recently published Phase III MIBC trials reporting results by HR: NIAGARA (perioperative durvalumab), CheckMate-274 (adjuvant nivolumab) and AMBASSADOR (adjuvant pembrolizumab). Using reconstructed individual patient data, RMST was calculated at a 42-month truncation. Costs were sourced from U.S. Medicare prices and calculated using each study’s median treatment duration.
Results
DFS-RMST differences at 42 months were: 3.39 months (95% CI: 1.4–5.39) for NIAGARA, 4.05 months (95% CI: 1.33–6.78) for CheckMate-274, and 3.97 months (95% CI: 1.3–6.65) for AMBASSADOR. OS-RMST differences were: 1.84 months (95% CI: 0.37–3.37) for NIAGARA, 2.56 months (95% CI: 0.54–4.58) for CheckMate-274, and 0.95 months (95% CI: –1.21 to 3.15) for AMBASSADOR. Cost per month of survival gained ranged from $43,460 to $49,832 for DFS and $52,387 to $207,374 for OS across trials.
Conclusion
Our RMST-based analysis highlights the minimal real-world survival benefits and provides more intuitive and clinically relevant interpretations than traditional hazard ratios. Our visualization framework offers an intuitive approach to assist clinicians and patients in making informed decisions.
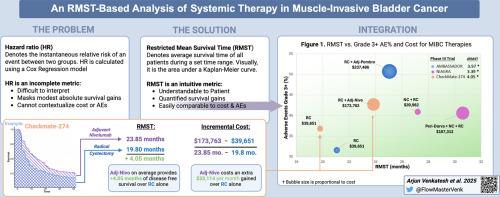
基于rmst的肌肉浸润性膀胱癌全身治疗分析。
导论:最近在围手术期使用免疫治疗药物治疗肌肉浸润性膀胱癌(MIBC)的III期试验重塑了治疗范式。传统的风险比虽然在统计上是可靠的,但可能掩盖了适度的绝对生存收益,使临床医生和患者的知情决策复杂化。本研究旨在利用限制平均生存时间(RMST)分析,为生存获益提供更直观的解释,并促进在三个关键试验中比较疗效、毒性和成本的框架的发展。方法:总生存期(OS)、无病生存期(DFS)和不良反应(AE)数据从最近发表的III期MIBC试验中提取,这些试验报告了HR: NIAGARA(围手术期durvalumab)、checkmate274(辅助nivolumab)和AMBASSADOR(辅助派姆单抗)的结果。使用重建的个体患者数据,在截断42个月时计算RMST。费用来源于美国医疗保险价格,并使用每个研究的中位治疗持续时间计算。结果:42个月时的DFS-RMST差异:NIAGARA为3.39个月(95% CI: 1.4-5.39), CheckMate-274为4.05个月(95% CI: 1.33-6.78), AMBASSADOR为3.97个月(95% CI: 1.3-6.65)。NIAGARA组OS-RMST差异为1.84个月(95% CI: 0.37-3.37), CheckMate-274组为2.56个月(95% CI: 0.54-4.58), AMBASSADOR组为0.95个月(95% CI: -1.21 - 3.15)。在所有试验中,DFS的每月生存成本从43460美元到49832美元不等,OS的每月生存成本从52387美元到207374美元不等。结论:我们基于rmst的分析强调了最小的现实生存益处,并提供了比传统风险比更直观和临床相关的解释。我们的可视化框架提供了一种直观的方法来帮助临床医生和患者做出明智的决定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.80
自引率
3.70%
发文量
297
审稿时长
7.6 weeks
期刊介绍:
Urologic Oncology: Seminars and Original Investigations is the official journal of the Society of Urologic Oncology. The journal publishes practical, timely, and relevant clinical and basic science research articles which address any aspect of urologic oncology. Each issue comprises original research, news and topics, survey articles providing short commentaries on other important articles in the urologic oncology literature, and reviews including an in-depth Seminar examining a specific clinical dilemma. The journal periodically publishes supplement issues devoted to areas of current interest to the urologic oncology community. Articles published are of interest to researchers and the clinicians involved in the practice of urologic oncology including urologists, oncologists, and radiologists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


