Assessing the uptake of core outcome sets in randomized controlled trials for localized prostate cancer: A cross-sectional study
IF 2.3
3区 医学
Q3 ONCOLOGY
Urologic Oncology-seminars and Original Investigations
Pub Date : 2025-07-30
DOI:10.1016/j.urolonc.2025.07.008
引用次数: 0
Abstract
Purpose
To evaluate core outcome set (COS) completion recommended in localized prostate cancer (LPC) randomized controlled trials (RCTs).
Methods
We identified the original LPC COS from 2017 established by the Core Outcome Measures in Effectiveness Trials Initiative. We conducted a search of LPC RCT registries between 2013 and 2023 from databases on ClinicalTrials.gov and International Clinical Trials Registry Platform (ICTRP) via who.int. We screened RCTs from our search in a masked, duplicate fashion. We extracted trial characteristics and specific COS outcomes for survival, bodily functions, quality of life, and treatment-specific outcomes again in a masked, duplicate fashion. COS uptake results were analyzed using an interrupted time series analysis.
Results
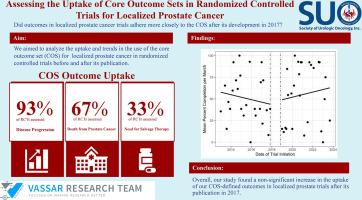
Our initial search of ClinicalTrials.gov and ICTRP yielded 13,909 trials. After exclusions, we extracted data from 82 clinical trials. "Disease Progression" (76/82; 92.68%) was the most commonly measured outcome while "Need for Salvage Therapy" (27/82; 32.93%) was the least. Limitations include lack of generalization for other COSs and inability to confirm systematic search returned all pertinent trials.
Conclusion
A nonsignificant decrease in COS adherence prior to the publication of a COS for LPC in 2017 occurred, then a subsequent nonsignificant increase in COS adherence after. We recommend LPC clinical trialists adhere to the COS outlined in our study and that further uptake studies be done to assess future LPC COS adherence.
评估局部前列腺癌随机对照试验中核心结局集的吸收:一项横断面研究。
目的:评价局限性前列腺癌(LPC)随机对照试验(rct)推荐的核心结局集(COS)完成情况。方法:我们确定了2017年由有效性试验倡议的核心结局指标建立的原始LPC COS。我们通过who.int从ClinicalTrials.gov和国际临床试验注册平台(International ClinicalTrials Registry Platform, ICTRP)的数据库中检索了2013年至2023年的LPC RCT注册。我们从我们的搜索中筛选随机对照试验,以掩盖,重复的方式。我们再次以一种隐藏的、重复的方式提取了试验特征和生存、身体功能、生活质量和治疗特异性结果的特定COS结果。使用中断时间序列分析COS摄取结果。结果:我们在ClinicalTrials.gov和ICTRP上的初步搜索获得了13909项试验。排除后,我们从82项临床试验中提取数据。“疾病进展”(76/82;92.68%)是最常见的测量结果,而“救助治疗需求”(27/82;32.93%)最少。局限性包括缺乏对其他COSs的泛化和无法确认系统搜索返回所有相关试验。结论:在2017年发表LPC的COS之前,COS依从性出现了不显著的下降,随后COS依从性出现了不显著的增加。我们建议LPC临床试验者坚持我们研究中概述的COS,并进行进一步的研究来评估未来LPC COS的依从性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.80
自引率
3.70%
发文量
297
审稿时长
7.6 weeks
期刊介绍:
Urologic Oncology: Seminars and Original Investigations is the official journal of the Society of Urologic Oncology. The journal publishes practical, timely, and relevant clinical and basic science research articles which address any aspect of urologic oncology. Each issue comprises original research, news and topics, survey articles providing short commentaries on other important articles in the urologic oncology literature, and reviews including an in-depth Seminar examining a specific clinical dilemma. The journal periodically publishes supplement issues devoted to areas of current interest to the urologic oncology community. Articles published are of interest to researchers and the clinicians involved in the practice of urologic oncology including urologists, oncologists, and radiologists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


