Cytoprotective role of resveratrol in cigarette smoke-induced pyroptosis through Nrf2 pathway activation
IF 3.2
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Resveratrol, a natural polyphenolic compound, has garnered increasing attention due to its antioxidant and anti-inflammatory properties. In this study, we investigated its protective role against cigarette smoke extract (CSE)-induced pyroptosis in human bronchial epithelial cell lines (BEAS-2B, 16HBE, and A549) and a chronic cigarette smoke (CS)-exposed mouse model. CS exposure is a major pathogenic factor in chronic obstructive pulmonary disease, primarily through promoting oxidative stress, inflammation, and pyroptotic cell death. Our results demonstrate that resveratrol enhances the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, upregulating downstream antioxidant enzymes such as HO-1 and NQO1. This activation mitigates oxidative stress and inhibits the activation of the TXNIP/NLRP3/caspase-1 inflammasome axis. In vitro, resveratrol reduced ROS accumulation and proinflammatory cytokine release in CSE-stimulated human bronchial epithelial cells. In vivo, resveratrol partially restored lung function and redox homeostasis in CS-exposed mice. Moreover, mechanistic analyses revealed that resveratrol upregulates miR-200a expression, which directly targets Keap1, thereby relieving its inhibition of Nrf2. These findings suggest that resveratrol alleviates CSE-induced pyroptosis by modulating the miR-200a/Keap1/Nrf2 axis and may serve as a potential therapeutic strategy for smoking-related airway diseases. However, additional clinical studies are necessary to confirm its efficacy.
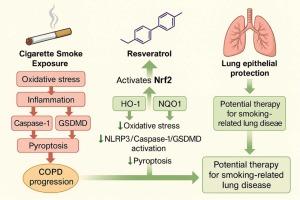
通过Nrf2通路激活白藜芦醇在香烟烟雾诱导的焦亡中的细胞保护作用。
白藜芦醇是一种天然的多酚类化合物,由于其抗氧化和抗炎特性而受到越来越多的关注。在这项研究中,我们研究了其对香烟烟雾提取物(CSE)诱导的人支气管上皮细胞系(BEAS-2B, 16HBE和A549)和慢性香烟烟雾暴露小鼠模型的保护作用。CS暴露是慢性阻塞性肺疾病(COPD)的一个主要致病因素,主要通过促进氧化应激、炎症和热腐细胞死亡。我们的研究结果表明,白藜芦醇增强Nrf2信号通路的激活,上调下游抗氧化酶如HO-1和NQO1。这种激活可以减轻氧化应激并抑制TXNIP/NLRP3/caspase-1炎症小体轴的激活。在体外,白藜芦醇减少了cse刺激的人支气管上皮细胞中ROS的积累和促炎细胞因子的释放。在体内,白藜芦醇部分恢复了cs暴露小鼠的肺功能和氧化还原稳态。此外,机制分析显示,白藜芦醇上调miR-200a的表达,miR-200a直接靶向Keap1,从而减轻其对Nrf2的抑制作用。这些发现表明,白藜芦醇通过调节miR-200a/Keap1/Nrf2轴减轻cse诱导的焦亡,可能作为吸烟相关气道疾病的潜在治疗策略。然而,需要更多的临床研究来证实其有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Stress & Chaperones
生物-细胞生物学
CiteScore
7.60
自引率
2.60%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Cell Stress and Chaperones is an integrative journal that bridges the gap between laboratory model systems and natural populations. The journal captures the eclectic spirit of the cellular stress response field in a single, concentrated source of current information. Major emphasis is placed on the effects of climate change on individual species in the natural environment and their capacity to adapt. This emphasis expands our focus on stress biology and medicine by linking climate change effects to research on cellular stress responses of animals, micro-organisms and plants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


