Oxygen isotopic re-equilibration during transformation of ikaite to calcite via amorphous calcium carbonate
IF 3.6
2区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
Abstract
Glendonites are calcite pseudomorphs of ikaite (CaCO3·6H2O), a mineral that typically forms below 4 °C. Ikaite is found in marine clastic sediments, caves and lacustrine sediments, and its presence is correlated with cold paleo-environments. However, it is uncertain whether the isotopic composition of glendonite preserves the low-temperature paleoclimate conditions. Here we study the ikaite to calcite transition by following the evolution of solutions containing ikaite, prepared at pH 12.1, 10.3 and 9.1, and filtered materials exposed to air. Ikaite transformation starts with the formation of 100–200 nm-sized amorphous calcium carbonate (ACC) on the surface of ikaite grains, which transforms to carbonates dominated by calcite. In Mg-free solutions, vaterite also forms, whereas low concentrations of Mg (e.g., 26 mg/l) favor aragonite formation. Our data suggest that synthetic ikaites are formed with strong 16O-enrichment relative to other carbonate species due to kinetic fractionation. The ikaite-derived carbonates formed in contact with ambient air show a minor δ13C change (−3.0 ± 0.8 ‰) relative to ikaite (−4.2 ± 0.3 ‰), but their oxygen isotope compositions remain constant. In contrast, transformation in solutions results in significant oxygen isotopic changes (from 17.7 to 20.5 ‰). Calcite-water oxygen isotopic equilibrium is approached within one week at 8 °C, and one day at 15 °C. Our experiments suggest that glendonite calcites formed by ikaite dehydration during early diagenesis undergo oxygen isotope exchange with pore waters and can record temperature and water composition conditions similar to those of the ikaite formation.
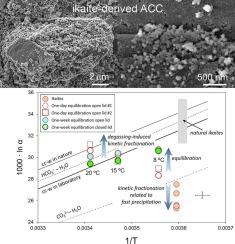
无定形碳酸钙使钛矿转变为方解石过程中的氧同位素再平衡
绿榴石是钙钛矿(CaCO3·6H2O)的方解石伪晶,通常形成于4℃以下。在海相碎屑沉积物、溶洞和湖相沉积物中均可发现爱凯特石,其存在与寒冷的古环境有关。然而,绿榴石的同位素组成是否保留了低温古气候条件尚不确定。本文通过研究在pH值为12.1、10.3和9.1的条件下制备的含钛矿溶液和暴露于空气中的过滤材料的演变,研究了钛矿向方解石的转变。易凯石相变始于在易凯石晶粒表面形成100 ~ 200 nm大小的无定形碳酸钙(ACC),并转变为以方解石为主的碳酸盐。在不含镁的溶液中,也会形成文石,而低浓度的镁(例如,26毫克/升)有利于文石的形成。我们的数据表明,相对于其他碳酸盐物种,合成风海岩由于动力学分馏而具有较强的16o富集。与环境空气接触形成的爱海岩型碳酸盐岩δ13C变化较小(- 3.0±0.8‰),而爱海岩型碳酸盐岩δ13C变化较小(- 4.2±0.3‰),但其氧同位素组成保持不变。相反,溶液中的转化导致氧同位素的显著变化(从17.7‰到20.5‰)。方解石-水氧同位素平衡在8°C下一周内达到,在15°C下一天达到。我们的实验表明,早期成岩过程中由冰碛石脱水形成的绿榴石方解石与孔隙水进行氧同位素交换,记录了与冰碛石形成时相似的温度和水组成条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Geology
地学-地球化学与地球物理
CiteScore
7.20
自引率
10.30%
发文量
374
审稿时长
3.6 months
期刊介绍:
Chemical Geology is an international journal that publishes original research papers on isotopic and elemental geochemistry, geochronology and cosmochemistry.
The Journal focuses on chemical processes in igneous, metamorphic, and sedimentary petrology, low- and high-temperature aqueous solutions, biogeochemistry, the environment and cosmochemistry.
Papers that are field, experimentally, or computationally based are appropriate if they are of broad international interest. The Journal generally does not publish papers that are primarily of regional or local interest, or which are primarily focused on remediation and applied geochemistry.
The Journal also welcomes innovative papers dealing with significant analytical advances that are of wide interest in the community and extend significantly beyond the scope of what would be included in the methods section of a standard research paper.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


