Both maternal and paternal genotypes modulate Wolbachia-induced cytoplasmic incompatibility in graham bean beetles
IF 3.9
2区 生物学
Q2 ECOLOGY
引用次数: 0
Abstract
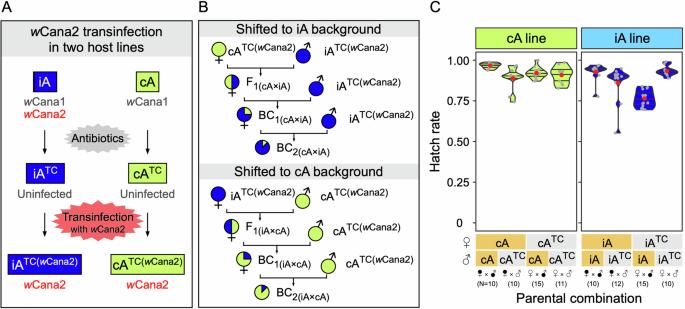
Cytoplasmic incompatibility (CI) is a phenomenon where embryonic development is disrupted–often leading to complete failure–when the female parent lacks the symbiont strain carried by the male parent. This mechanism, employed by maternally transmitted symbionts such as Wolbachia, facilitates their rapid spread within a host population. CI has significant potential as a tool for achieving population replacement or suppression, particularly targeting disease vectors and agricultural pests. While complete expression of CI is ideal for such applications, its intensity can vary. Despite extensive research on the symbiont genotypes, the influence of host genetic background on CI expression remains poorly understood. Here, we found that in the bean beetle Callosobruchus analis, the Wolbachia strain wCana2 induces weak CI in its native nuclear background but strong CI in a previously unassociated nuclear background. Crossing experiments reveal that the nuclear backgrounds of both male and female parents can significantly affect CI expression independent of Wolbachia titres in C. analis. These findings uncover novel perspectives on the host-symbiont interactions underlying CI and highlight the complexities to be addressed for its practical application.
母系和父系基因型均可调节沃尔巴克氏体诱导的格雷厄姆豆甲虫细胞质不亲和性。
细胞质不相容(Cytoplasmic incompatibility, CI)是一种胚胎发育被破坏的现象,通常导致胚胎发育完全失败,因为雌性亲本缺乏雄性亲本携带的共生菌株。这种由母体传播的共生体(如沃尔巴克氏体)采用的机制促进了它们在宿主种群中的快速传播。CI作为实现种群替代或抑制的工具具有巨大潜力,特别是针对病媒和农业害虫。虽然完整的CI表达对于此类应用程序是理想的,但其强度可能有所不同。尽管对共生体基因型进行了广泛的研究,但宿主遗传背景对CI表达的影响仍然知之甚少。在这里,我们发现在豆甲虫Callosobruchus分析中,Wolbachia菌株wCana2在其原生核背景中诱导弱CI,而在先前不相关的核背景中诱导强CI。杂交实验表明,雌雄亲本的核背景均能显著影响C. analis中独立于沃尔巴克氏体滴度的CI表达。这些发现揭示了宿主-共生体相互作用的新视角,并突出了其实际应用中需要解决的复杂性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heredity
生物-进化生物学
CiteScore
7.50
自引率
2.60%
发文量
84
审稿时长
4-8 weeks
期刊介绍:
Heredity is the official journal of the Genetics Society. It covers a broad range of topics within the field of genetics and therefore papers must address conceptual or applied issues of interest to the journal''s wide readership
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


