Comparative Performance of Calcium Phosphate Grafts and Iliac Crest Autograft in Posterolateral Spinal Fusion in Rabbits
Abstract
Background
Calcium phosphate (CaP) biomaterials are widely used in surgical applications such as spinal fusion to substitute for or extend autogenous bone graft. Preclinical testing in standardized animal models is useful for evaluating the relative performance of materials differing in composition and structure, including a newer generation of submicron-structured CaP (sCaP) with surface features uniformly smaller than 1 μm. The purpose of this study was to compare three clinically available CaP-based materials and iliac crest autograft in the rabbit posterolateral fusion (PLF) model.
Methods
A novel sCaP with bovine collagen type I (sCaP/Col I) and two clinically established materials, sCaP with alkylene oxide copolymer (sCaP/AOC) and microstructured CaP with bovine collagen type I (mCaP/Col I), were evaluated in a skeletally mature, single-level, non-instrumented, bilateral rabbit PLF model. Iliac crest autograft served as a control. Endpoints included radiographic, mechanical, and histological evaluation at postoperative 6, 9, and 12 weeks.
Results
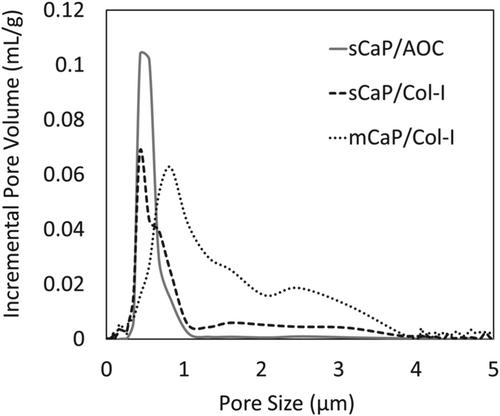
Fusion progressed with postoperative time with all grafts, and the CaP materials yielded fusion rates by micro-CT and manual palpation similar to those of the autograft control at each time point. When tested as autograft extenders, sCaP/Col I and sCaP/AOC demonstrated equivalent results for all endpoints. When hydrated with bone marrow aspirate and used as bone graft substitutes, sCaP/Col I supported earlier fusion than mCaP/Col I with an increased radiographic fusion rate at 9 weeks (p = 0.032) and increased bone tissue content by histomorphometry at 12 weeks (p = 0.006). New bone was observed to form with all materials, and no adverse local biological reactions were seen.
Conclusions
Differences in the composition and structure of clinically available CaP-based materials influenced the achievement of spinal fusion in a standardized rabbit PLF model. These results may help guide the selection and use of materials in clinical applications and the future development of biomaterials with improved performance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


