N2O2 Schiff-Base nickel(II) and iron(III) template complexes of 2-hydroxy-5-ethoxyacetophenone S-methylthiosemicarbazone: Synthesis, characterization, DFT calculations, molecular docking, and antioxidant activity
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Two new complexes, [Ni (L)] (1) and [Fe (L)Cl] (2), were synthesized through template condensation of 2-hydroxy-5-ethoxyacetophenone S-methylthiosemicarbazone (H2L1) with 2-hydroxy-4-methoxybenzaldehyde in the presence of nickel(II) and iron(III) ions. The complexes were characterized using elemental analysis, magnetic measurements, FT-IR, UV–Vis, ESI-MS and 1H NMR spectra. In order to gain further insights into their structural features, Density Functional Theory (DFT) calculations were employed to optimize the geometries and predict vibrational frequencies of the complexes, providing valuable insights into their structural stability and bonding interactions. The frontier molecular orbital (HOMO and LUMO) analyses were conducted for the optimized geometries to evaluate the electronic properties and chemical reactivity. The computational results confirmed the proposed square-planar geometry for (1) and square-pyramidal geometry for (2). TD-DFT analyses predicted the theoretical UV–Vis spectra and compared with experimental spectra. Molecular docking analyses were performed with EGFR (PDB ID: 4HJO) and B-DNA (PDB ID: 1BNA). Binding parameters and ADME analyses were also conducted. The antioxidant capacity of the compounds were examined using the CUPRAC (cupric reducing antioxidant capacity) method, and their radical scavenging activity was determined by the DPPH (1,1-Diphenyl-2-picrylhydrazyl) assay. [Ni(L)] exhibited higher antioxidant activity compared to [Fe(L)Cl] and the ligand (H2L1) in the CUPRAC assay.
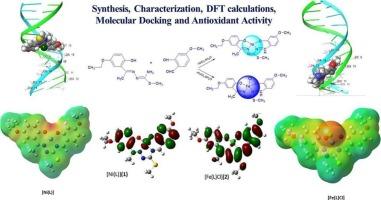
N2O2希夫碱镍(II)和铁(III)模板配合物2-羟基-5-乙氧基苯乙酮s -甲基硫代氨基脲:合成、表征、DFT计算、分子对接和抗氧化活性
在镍(II)和铁(III)离子存在下,用2-羟基-5-乙氧基苯乙酮s -甲基硫代氨基脲(H2L1)与2-羟基-4-甲氧基苯甲醛模板缩合合成了两个新的配合物[Ni (L)](1)和[Fe (L)Cl](2)。通过元素分析、磁性测量、FT-IR、UV-Vis、ESI-MS和1H NMR对配合物进行了表征。为了进一步了解它们的结构特征,研究人员利用密度泛函理论(DFT)计算优化了这些配合物的几何形状,并预测了它们的振动频率,从而对它们的结构稳定性和键相互作用提供了有价值的见解。通过前沿分子轨道(HOMO和LUMO)分析,对优化后的结构进行了电子性能和化学反应性评价。计算结果证实了(1)的方平面几何和(2)的方锥体几何。TD-DFT分析预测了理论紫外-可见光谱,并与实验光谱进行了比较。与EGFR (PDB ID: 4HJO)和B-DNA (PDB ID: 1BNA)进行分子对接分析。并进行了结合参数和ADME分析。采用CUPRAC(铜还原抗氧化能力)法检测化合物的抗氧化能力,采用DPPH(1,1-二苯基-2-苦味酰肼)法测定化合物的自由基清除能力。在CUPRAC实验中,[Ni(L)]表现出比[Fe(L)Cl]和配体(H2L1)更高的抗氧化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


