Solubility and dissolution kinetics of uranium trifluoride in 2LiF-BeF2 molten salt
IF 3.2
2区 工程技术
Q3 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Although uranium trifluoride (UF3) holds promise for applications in molten salt reactors, its high-temperature physicochemical properties in molten salt systems have yet to be thoroughly explored. In this study, the solubility and dissolution kinetics of UF3 in molten 2LiF-BeF2 (66–34 mol %, FLiBe) eutectic salt were investigated using the isothermal saturation method within the temperature range of 823 K to 973 K. High-purity UF3 compacts (∼99.70 %) were synthesized via an optimized solid-phase reaction protocol and subsequent compacting. The UF3-saturated FLiBe molten salts were prepared by immerse dissolution of nickel mesh-wrapped UF3 compacts and bulk uranium in the molten salt, eliminating the filtration step. Experimental findings showed that the dissolution equilibrium of UF3 in the FLiBe salt was 120 h. The solubility of UF3 exhibited a linear increase (R2 > 0.99) from 4.16 wt. % to 12.60 wt. % as the elevation of temperature. Crystallographic analysis confirmed that the typical UF3 phase was the only uranium-bearing phase throughout the dissolution process, while deconvolution X-ray photoelectron spectroscopy (XPS) verified the exclusive presence of the U3+ species under all temperature conditions. Notably, the dissolution kinetics conformed to a mass transport control model.
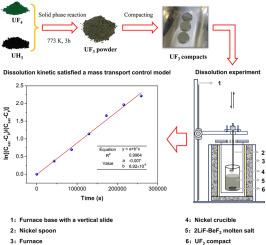
三氟化铀在2LiF-BeF2熔盐中的溶解度和溶解动力学
虽然三氟化铀(UF3)有望应用于熔盐反应堆,但其在熔盐系统中的高温物理化学性质尚未得到彻底探索。在823 ~ 973 K的温度范围内,采用等温饱和法研究了UF3在熔融2LiF-BeF2 (66-34 mol %, FLiBe)共晶盐中的溶解度和溶解动力学。通过优化的固相反应方案和随后的压实,合成了高纯度UF3压实剂(~ 99.70%)。通过将镍网包裹的UF3压片和大块铀浸入溶解在熔盐中,消除了过滤步骤,制备了饱和UF3的FLiBe熔盐。实验结果表明,UF3在FLiBe盐中的溶解平衡为120 h,其溶解度呈线性增加(R2 >;0.99)从4.16重量%到12.60重量%,随着温度的升高。晶体学分析证实了典型的UF3相是整个溶解过程中唯一的含铀相,而反卷积x射线光电子能谱(XPS)证实了在所有温度条件下U3+的专属存在。值得注意的是,溶解动力学符合质量传递控制模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nuclear Materials
工程技术-材料科学:综合
CiteScore
5.70
自引率
25.80%
发文量
601
审稿时长
63 days
期刊介绍:
The Journal of Nuclear Materials publishes high quality papers in materials research for nuclear applications, primarily fission reactors, fusion reactors, and similar environments including radiation areas of charged particle accelerators. Both original research and critical review papers covering experimental, theoretical, and computational aspects of either fundamental or applied nature are welcome.
The breadth of the field is such that a wide range of processes and properties in the field of materials science and engineering is of interest to the readership, spanning atom-scale processes, microstructures, thermodynamics, mechanical properties, physical properties, and corrosion, for example.
Topics covered by JNM
Fission reactor materials, including fuels, cladding, core structures, pressure vessels, coolant interactions with materials, moderator and control components, fission product behavior.
Materials aspects of the entire fuel cycle.
Materials aspects of the actinides and their compounds.
Performance of nuclear waste materials; materials aspects of the immobilization of wastes.
Fusion reactor materials, including first walls, blankets, insulators and magnets.
Neutron and charged particle radiation effects in materials, including defects, transmutations, microstructures, phase changes and macroscopic properties.
Interaction of plasmas, ion beams, electron beams and electromagnetic radiation with materials relevant to nuclear systems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


