Characterization of conformation-specific antibodies TOMA-1 and TTCM-1 for recombinant tau monomer and amplified brain derived tau oligomer
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-07-28
DOI:10.1016/j.bbadis.2025.167995
引用次数: 0
Abstract
Tauopathies are a set of neurodegenerative diseases characterized by the pathological accumulation of aggregated tau in the brain. Recent breakthrough evidence has revealed the existence of different strains of tau oligomer (TauO) which direct the different pathological presentation of individual tauopathies. Extensive research efforts have been devoted to search for specific antibodies or drug candidates which target TauO to serve as promising alternatives to treat Alzheimer's disease (AD) in future. To screen for the antibodies which are able to bind with amplified brain derived tau oligomer (aBDTO), we have investigated the binding parameters of the tau oligomer-specific monoclonal antibody-1 (TOMA-1) and toxic tau conformation-specific monoclonal antibody-1 (TTCM-1) with the recombinant tau monomer (rTauM) and aBDTO using isothermal titration calorimetry (ITC). TOMA-1 specifically recognizes the amino acid sequences 367–386, 382–401 and 367–386 and TTCM-1 specifically recognizes the amino acid sequence 307–326 of rTauM and aBDTO, respectively. Our results demonstrated that both TOMA-1 and TTCM-1 have a high binding affinity with aBDTO compared to rTauM. We also observed that higher the binding affinity of the antibody to the aBDTO, lower was the toxicity of the aBDTO and vice versa. Our study taken together presents both TOMA-1 and TTCM-1 to be potential immunotherapeutic agents against AD.
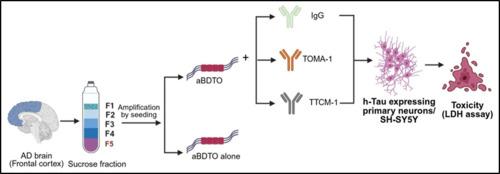
重组tau单体和扩增脑源性tau寡聚物构象特异性抗体TOMA-1和TTCM-1的鉴定
tau病是一组神经退行性疾病,其特征是大脑中聚集的tau的病理积累。最近的突破性证据揭示了不同菌株的tau寡聚物(TauO)的存在,它们指导了个体tau病的不同病理表现。广泛的研究工作致力于寻找针对TauO的特异性抗体或候选药物,以作为未来治疗阿尔茨海默病(AD)的有希望的替代方案。为了筛选能够与扩增脑源性tau寡聚物(aBDTO)结合的抗体,我们利用等温滴定量热法(ITC)研究了tau寡聚物特异性单克隆抗体-1 (TOMA-1)和毒性tau构象特异性单克隆抗体-1 (TTCM-1)与重组tau单体(rTauM)和aBDTO的结合参数。TOMA-1特异性识别rTauM和aBDTO的367-386、382-401和367-386氨基酸序列,TTCM-1特异性识别rTauM和aBDTO的307-326氨基酸序列。我们的研究结果表明,与rTauM相比,TOMA-1和TTCM-1与aBDTO具有较高的结合亲和力。我们还观察到抗体与aBDTO的结合亲和力越高,aBDTO的毒性越低,反之亦然。我们的研究表明,TOMA-1和TTCM-1都是潜在的AD免疫治疗剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


