Depression- and exercise-associated stimuli exert contrasting effects on neural stem cell activity and paracrine signaling
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-07-31
DOI:10.1016/j.bbadis.2025.167997
引用次数: 0
Abstract
Adult neurogenesis is dysregulated in neurological disorders, including depression. Adult neural stem cells (NSCs) are close to the vasculature and the cerebrospinal fluid, placing them in an ideal position to receive extrinsic signals and transmit these cues to the neurogenic niche. Herein, we aimed to explore how different systemic cues influence the regenerative properties of NSC secretome on recipient differentiating cells and microglia, key neurogenic components. To mimic signals that NSCs may sense in pathological conditions, we used the secretome of oxidative damaged cells (acute oxidative damage), and the serum from depressed mice (depression-associated chronic signals). Alternatively, NSCs were conditioned with different mitochondrial metabolic regulators to mimic a pro-metabolic NSC environment. Results showed that both injury and metabolic stimuli triggered the increase of NSC proliferation and mitochondrial fragmentation, along with the delivery of a neuroprotective secretome toward injured recipient cells. However, premature differentiation was only observed in NSCs sensing depression-associated chronic signals. The secretome from metabolic-stimulated NSCs favored neurogenesis of target cells, being enriched in regenerative metabolites. Depression-associated signals promoted a NSC secretome with reduced regenerative metabolites and microRNAs that repressed microglial phagocytosis and differentiation in target NSCs. At last, the reduction of oxidative phosphorylation-related proteins in the neurogenic niche of depressed mice was rescued by physical exercise. Our data indicate a central role of external metabolic and injury signals in regulating the neurogenic niche through NSC paracrine activity, unveiling distinct NSC regenerative responses upon transient acute and chronic injuries, and new cues for physical exercise-induced alleviation of depression.
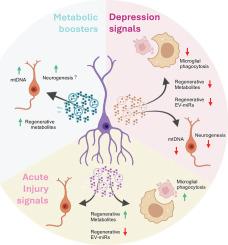
抑郁和运动相关的刺激对神经干细胞活性和旁分泌信号产生不同的影响
成人神经发生在包括抑郁症在内的神经系统疾病中是失调的。成体神经干细胞(NSCs)靠近脉管系统和脑脊液,使它们处于理想的位置,可以接收外部信号并将这些信号传递给神经源性生态位。在此,我们旨在探讨不同的系统线索如何影响NSC分泌组对受体分化细胞和小胶质细胞(关键神经源性成分)的再生特性。为了模拟NSCs在病理状态下可能感知的信号,我们使用了氧化损伤细胞(急性氧化损伤)的分泌组和抑郁小鼠的血清(抑郁相关的慢性信号)。另外,用不同的线粒体代谢调节剂来调节NSCs,以模拟促代谢的NSC环境。结果表明,损伤和代谢刺激均触发NSC增殖和线粒体断裂的增加,并向受伤的受体细胞传递神经保护性分泌组。然而,仅在感知抑郁相关慢性信号的NSCs中观察到过早分化。代谢刺激的NSCs分泌组有利于靶细胞的神经发生,富含再生代谢物。抑郁相关信号促进NSC分泌组减少再生代谢物和microrna,从而抑制目标NSCs的小胶质细胞吞噬和分化。最后,通过体育锻炼可以挽救抑郁小鼠神经源性生态位中氧化磷酸化相关蛋白的减少。我们的数据表明,外部代谢和损伤信号在通过NSC旁分泌活动调节神经源性生态位中的核心作用,揭示了短暂急性和慢性损伤时不同的NSC再生反应,以及体育锻炼引起的抑郁症缓解的新线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


