Combining MCL-1 inhibition and CD37-directed chimeric antigen receptor T cells as an effective strategy to target T-cell lymphoma
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
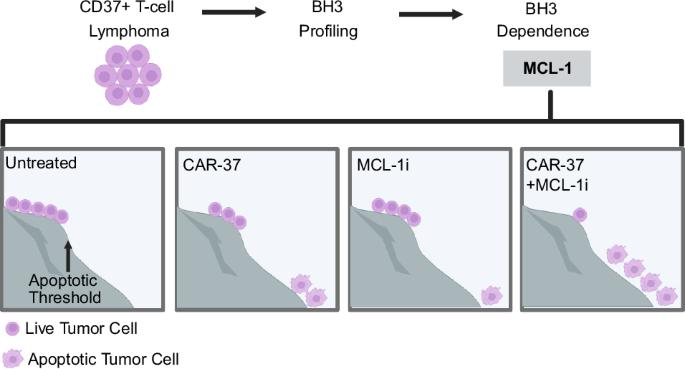
Chimeric antigen receptor (CAR) T cell therapy has not yet been realized for T-cell lymphomas (TCL), partially due to challenges in identifying tumor-specific antigens. We previously reported selective expression of CD37 on malignant T cells in a subset of TCL. Herein, we demonstrate CAR-37 T cells specifically target CD37-positive TCL in part by activating the intrinsic apoptotic pathway. To maximize therapeutic index, we identified selective/targetable BH3 dependences in individual TCL models and combined with CAR-37 T cells. We show that BH3 mimetics do not alter CD37 antigen binding capacity on TCL and have minimal effects on CAR-37 T-cell phenotype or function. In TCL models with dependence on MCL-1, combining CAR-37 T cells and the MCL-1 inhibitor AZD5991 increases anti-TCL response and prolongs survival of xenografted mice. These findings suggest that personalized selection of BH3 mimetic/CAR-T combinations could maximize the therapeutic index for patients with TCL and possibly other diseases.
MCL-1抑制与cd37靶向嵌合抗原受体T细胞联合作为靶向T细胞淋巴瘤的有效策略
嵌合抗原受体(CAR) T细胞治疗尚未实现T细胞淋巴瘤(TCL),部分原因是在识别肿瘤特异性抗原方面存在挑战。我们之前报道了CD37在TCL亚型的恶性T细胞上的选择性表达。在此,我们证明car - 37t细胞通过激活内在凋亡途径特异性靶向cd37阳性TCL。为了最大限度地提高治疗指数,我们在单个TCL模型中鉴定了选择性/靶向BH3依赖性,并与CAR-37 T细胞联合使用。我们发现BH3模拟物不会改变CD37抗原在TCL上的结合能力,对CAR-37 t细胞表型或功能的影响最小。在依赖MCL-1的TCL模型中,CAR-37 T细胞与MCL-1抑制剂AZD5991联合使用可增加抗TCL反应,延长异种移植小鼠的存活时间。这些发现表明,个性化选择BH3模拟物/CAR-T组合可以最大限度地提高TCL患者的治疗指数,也可能是其他疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


