PABPN1-C5 axis promotes hepatocellular carcinoma progression via NF-κB activation
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
RNA polyadenylation is a key post-transcriptional modification essential for gene expression regulation. However, the role and mechanism of polyadenylation and its key molecule, polyadenylate binding protein nuclear 1 (PABPN1), in hepatocellular carcinoma (HCC) remain poorly understood. This study investigates the role of PABPN1 and its regulatory genes in HCC progression to identify potential therapeutic targets. Analysis of The Cancer Genome Atlas (TCGA) dataset and an independent HCC cohort revealed significant upregulation of PABPN1 in HCC patients, which correlates with poor prognosis. Loss-of-function studies using HCC cell lines and conditional knockout mouse models demonstrated that targeting PABPN1 inhibited HCC progression. Conversely, overexpression of PABPN1 promoted HCC development in vitro and in a hydrodynamic transfection hepatocarcinogenesis mouse model. Mechanistic investigations showed that PABPN1 modulates C5 mRNA polyadenylation and stability, with the PABPN1-C5 axis driving NF-κB activation and recruiting polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) to promote HCC progression. Therapeutic targeting of the PABPN1-C5 axis using the C5a receptor inhibitor CCX168 significantly inhibited HCC progression in both in vitro and in vivo models. This study identifies PABPN1 as a critical regulator of HCC development and sheds light on the post-transcriptional regulation of complement components in cancer. Targeting the PABPN1-C5 axis represents a promising strategy for HCC treatment.
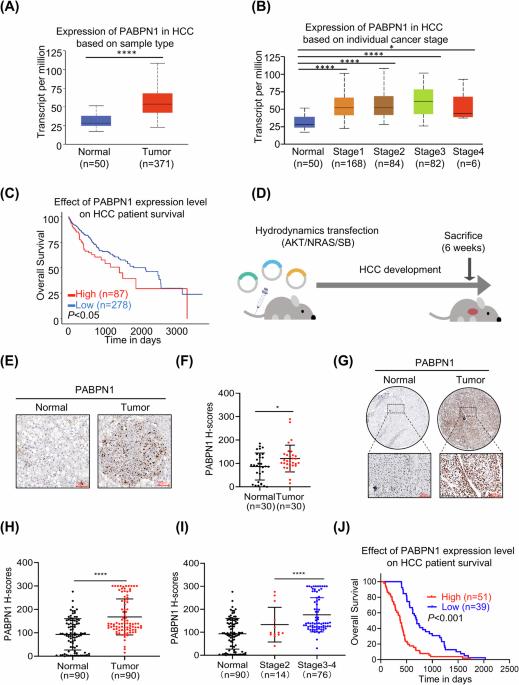
PABPN1-C5轴通过NF-κB活化促进肝细胞癌进展。
RNA多聚腺苷化是基因表达调控的关键转录后修饰。然而,聚腺苷酸化及其关键分子聚腺苷酸结合蛋白核1 (PABPN1)在肝细胞癌(HCC)中的作用和机制尚不清楚。本研究探讨了PABPN1及其调控基因在HCC进展中的作用,以确定潜在的治疗靶点。Cancer Genome Atlas (TCGA)数据集和独立HCC队列分析显示,PABPN1在HCC患者中显著上调,与预后不良相关。使用HCC细胞系和条件敲除小鼠模型进行的功能丧失研究表明,靶向PABPN1可抑制HCC进展。相反,在体外和水动力转染肝癌小鼠模型中,PABPN1的过表达促进了HCC的发展。机制研究表明,PABPN1调节C5 mRNA多聚腺苷化和稳定性,PABPN1-C5轴驱动NF-κB活化并募集多形核髓源性抑制细胞(PMN-MDSCs)促进HCC进展。在体外和体内模型中,使用C5a受体抑制剂CCX168靶向治疗PABPN1-C5轴可显著抑制HCC进展。这项研究确定了PABPN1是HCC发展的关键调节因子,并揭示了癌症中补体成分的转录后调节。靶向PABPN1-C5轴是一种很有前景的HCC治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


