EWS::FLI1-DHX9 interaction promotes Ewing sarcoma sensitivity to DNA topoisomerase 1 poisons by altering R-loop metabolism
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
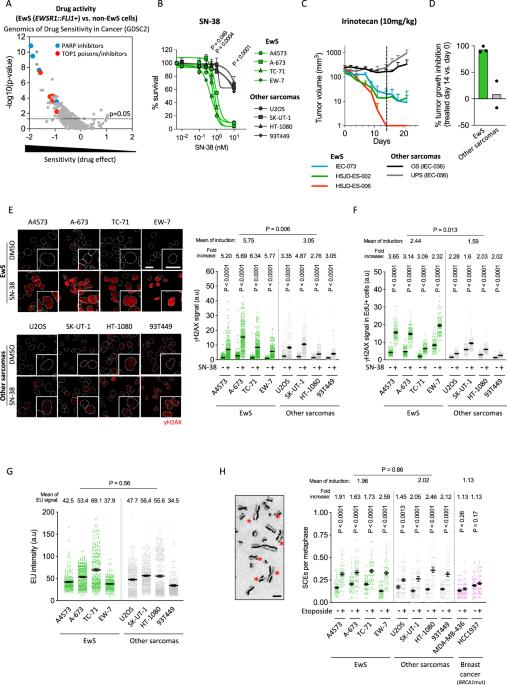
Drug resistance is an ill-defined cause of dismal outcomes in cancer. Ewing sarcoma (EwS), a pediatric cancer characterized by high therapy failure rates, is driven by a single oncogenic event generating EWSR1::ETS gene fusions (primarily EWSR1::FLI1) in a silent genomic background. This provides a straightforward model to study the impact of gene fusions on drug responses. Here, we describe a novel mechanism of sensitivity to DNA topoisomerase 1 poisons in EwS. We discovered that EWS::FLI1 prevents the resolution of R-loops induced by these drugs via sequestering DHX9 helicase, ultimately resulting in R-loop accumulation, replication stress, and genome instability. In turn, excessive DHX9 or reduced EWS::FLI1 levels render EwS cells resistant to the active metabolite of irinotecan (SN-38) independent of proliferation and global transcription rates. This resistance helps explain how elevated DHX9 levels predict worse clinical outcomes. Overall, our research demonstrates the impact of a dominant mutation on cancer drug sensitivity, highlighting its significant clinical implications.
EWS::FLI1-DHX9相互作用通过改变r环代谢促进Ewing肉瘤对DNA拓扑异构酶1毒物的敏感性。
耐药性是导致癌症预后不佳的一个不明确原因。Ewing肉瘤(EwS)是一种以高治疗失败率为特征的儿科癌症,它是由一个单一的致癌事件驱动的,在沉默的基因组背景下产生EWSR1::ETS基因融合(主要是EWSR1::FLI1)。这为研究基因融合对药物反应的影响提供了一个简单的模型。在这里,我们描述了EwS对DNA拓扑异构酶1毒物敏感的新机制。我们发现EWS::FLI1通过隔离DHX9解旋酶阻止这些药物诱导的r -环的分解,最终导致r -环积累、复制应激和基因组不稳定。反过来,过量的DHX9或降低的EWS::FLI1水平使EWS细胞对伊立替康(SN-38)的活性代谢物产生耐药性,而不依赖于增殖和全球转录率。这种耐药性有助于解释DHX9水平升高如何预示更糟糕的临床结果。总的来说,我们的研究证明了显性突变对癌症药物敏感性的影响,突出了其重要的临床意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


