CGRP+ fibers sprout within gastrocnemius muscle following complete spinal cord injury in rodents
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Following spinal cord injury nociceptive afferents exhibit functional and anatomical plasticity within the spinal dorsal horn. This plasticity is, at least in part, maladaptive, contributing to secondary complications related to neuropathic pain, autonomic dysreflexia, and spasticity. While previous studies have shown that nociceptors, particularly CGRP+ C-fibers, are also capable of sprouting peripherally following pathologies of chronic inflammation, muscle disuse, and denervation, the peripheral plasticity of C-fibers following spinal cord injury remains largely uninvestigated. Here, we show that CGRP+ nerve fibers, likely nociceptors, display expanded innervation within the gastrocnemius muscle, particularly near blood vessels and within the calcaneal tendon. Furthermore, CGRP+ sensory axons exhibit a fundamental change in their innervation pattern near motor axons, innervating independently of, rather than along, motor trunks. These changes occurred alongside significantly reduced size and expression of CGRP by motor axon terminals following spinal transection. We also report positive correlations between volume of CGRP+ fibers in the gastrocnemius muscle and muscle atrophy, hypersensitivity, intraspinal plasticity, and activation of glia within the lumbar spinal cord. Importantly, bulk RNA sequencing of muscle revealed upregulation of genes indicative of biological processes occurring at 6 weeks post-SCI that may contribute to sensory plasticity including inflammation, immune cell infiltration, and apoptosis. Taken together, these data suggest that many nociceptor-dependent complications that are thought to be spinally mediated may be driven or exacerbated by nociceptor plasticity occurring in the periphery.
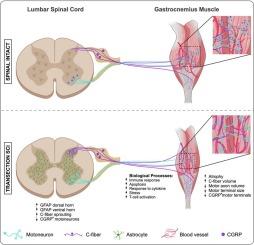
CGRP+纤维在啮齿类动物完全性脊髓损伤后腓肠肌内萌发。
脊髓损伤后,脊髓背角内的伤害性传入事件表现出功能和解剖上的可塑性。这种可塑性至少在一定程度上是不适应的,导致了与神经性疼痛、自主神经反射障碍和痉挛相关的继发性并发症。虽然先前的研究表明,伤害感受器,特别是CGRP+ c -纤维,也能够在慢性炎症、肌肉失用和去神经支配病理后外周生长,但脊髓损伤后c -纤维的外周可塑性仍未得到充分研究。在这里,我们发现CGRP+神经纤维,可能是伤害感受器,在腓肠肌内显示出扩张的神经支配,特别是在血管附近和跟腱内。此外,CGRP+感觉轴突在运动轴突附近表现出其神经支配模式的根本变化,独立于运动干而不是沿运动干支配。这些变化与脊髓横断后运动轴突末端CGRP的大小和表达显著降低同时发生。我们还报道了腓肠肌中CGRP+纤维的体积与肌肉萎缩、过敏、椎管内可塑性和腰椎神经胶质细胞激活之间的正相关。重要的是,肌肉的大量RNA测序显示,在脊髓损伤后6 周,指示生物过程的基因上调,可能有助于感觉可塑性,包括炎症、免疫细胞浸润和凋亡。综上所述,这些数据表明,许多被认为是脊髓介导的伤害感受器依赖性并发症可能被外周发生的伤害感受器可塑性所驱动或加剧。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


