Structural and Functional Characterization of Pseudomonas aeruginosa Virulence Factor AaaA, an Autotransporter with Arginine-Specific Aminopeptidase Activity
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
AaaA is a virulence-associated outer membrane protein found in the Gram-negative pathogen Pseudomonas aeruginosa. Classified as both an autotransporter and a member of the M28 family of aminopeptidases, AaaA has been shown to cleave N-terminal arginine residues from host-derived peptides. This activity has been demonstrated to enhance bacterial survival and suppress host immune responses by increasing local arginine availability. Here, we report the first successful purification and combined structural and biochemical characterization of full-length AaaA. We resolved its cryo-EM structure at 3.87 Å resolution, revealing the canonical three-domain architecture of autotransporters: a signal peptide, a passenger domain, and a translocator domain. Notably, the passenger domain adopts a compact globular fold characteristic of M28 aminopeptidases, which is less common than the extended or β-helical structures observed in the majority of autotransporters structurally characterized to date. The structure reveals a zinc-coordinated catalytic site and a negatively charged substrate binding pocket, consistent with specificity for positively charged N-terminal arginine residues. Mutagenesis of active site residues confirmed the molecular basis for arginine recognition. Functional assays demonstrated that AaaA exhibits zinc-dependent aminopeptidase activity across a broad pH (6–10) and temperature (20–60 °C) range. Together, these findings provide fundamental insights into the structure and function of AaaA and establish a framework for future efforts to develop targeted inhibitors that may attenuate P. aeruginosa virulence.
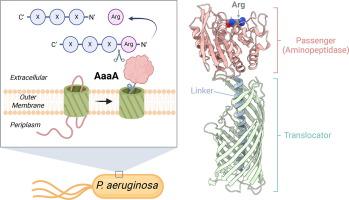
具有精氨酸特异性氨基肽酶活性的铜绿假单胞菌毒力因子AaaA的结构和功能特征。
AaaA是在革兰氏阴性病原体铜绿假单胞菌中发现的一种毒力相关的外膜蛋白。AaaA被归类为自转运蛋白和M28氨基肽酶家族的成员,已被证明可以从宿主衍生的肽中切割n端精氨酸残基。这种活性已被证明通过增加局部精氨酸可用性来提高细菌存活率和抑制宿主免疫反应。在这里,我们报道了首次成功纯化并结合结构和生化表征全长AaaA。我们以3.87 Å分辨率解析了其冷冻电镜结构,揭示了典型的三域结构:信号肽,乘客结构域和转运结构域。值得注意的是,乘客结构域采用M28氨基肽酶的致密球状褶皱特征,这比迄今为止在大多数结构表征的自转运蛋白中观察到的延伸或β-螺旋结构更少见。该结构揭示了一个锌配位催化位点和一个带负电的底物结合袋,与带正电的n端精氨酸残基的特异性一致。活性位点残基的突变证实了精氨酸识别的分子基础。功能分析表明,AaaA在较宽的pH值(6-10)和温度(20-60°C)范围内表现出锌依赖性氨基肽酶活性。总之,这些发现为AaaA的结构和功能提供了基本的见解,并为未来开发可能减弱铜绿假单胞菌毒力的靶向抑制剂建立了框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


