Icaritin protects against airway inflammation by inhibiting the TLR4/NF-κB pathway in vivo and in vitro
IF 2.8
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Icaritin, a bioactive phytomolecule derived from Epimedium flavonoids (EFs), has been shown to have anti-inflammatory, anti-proliferative, and pro-apoptotic properties. However, its potential mechanisms in asthma airway inflammation have not been elucidated. In this study, Ovalbumin (OVA)-induced asthma mouse model and human bronchial epithelial cells (BEAS-2B) were used to illustrate the effects and mechanisms of Icaritin on airway inflammation. Specific airway resistance (sRAW) was used to detect the airway hyperresponsiveness (AHR). Hematoxylin-eosin (H&E) and periodic acid schiff (PAS) were used to detect the pathological changes. Bronchoalveolar lavage fluid (BALF) was used to detect the airway inflammatory cells. Serum and supernatants were used to detect the cytokines. Immunohistochemistry (IHC) and western blotting were used to detect the expression of TLR4, p-65, p-p65, IκBα, and p-IκBα. Cell Counting Kit-8 (CCK-8) was used to detect the cell viability. Icaritin suppressed AHR, attenuated eosinophilic infiltration and mucus hypersecretion, and significantly reduced the levels of OVA-specific cytokines in asthmatic mice. Moreover, Icaritin inhibited TLR4 expression, decreased phosphorylation of IκBα, and reduced NF-κB p65 activation in lung tissue of asthmatic mice. Further mechanistic studies showed that Icaritin reduces TLR4-induced inflammatory factor expression and blocks TLR4-activated NF-κB pathway in BEAS-2B cells. These findings demonstrate for the first time that Icaritin suppresses airway inflammation in asthma by inhibiting the TLR4/NF-κB pathway, suggesting its potential as a therapeutic agent for asthma.
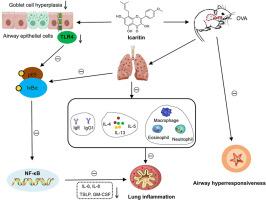
在体内和体外实验中,淫羊藿苷通过抑制TLR4/NF-κB通路对气道炎症具有保护作用
淫羊藿黄酮类化合物淫羊藿苷(Icaritin)是淫羊藿黄酮类化合物中的一种生物活性植物分子,具有抗炎、抗增殖和促细胞凋亡的作用。然而,其在哮喘气道炎症中的潜在机制尚未阐明。本研究通过卵清蛋白(OVA)诱导的哮喘小鼠模型和人支气管上皮细胞(BEAS-2B),探讨了淫羊藿苷对气道炎症的影响及其机制。采用特异性气道阻力(sRAW)检测气道高反应性(AHR)。采用苏木精-伊红(H&;E)和周期性酸席夫(PAS)检测病理变化。支气管肺泡灌洗液(BALF)检测气道炎症细胞。血清和上清液检测细胞因子。采用免疫组化(IHC)和western blotting检测TLR4、p-65、p-p65、i - κ b α、p- i - κ b α的表达。细胞计数试剂盒-8 (CCK-8)检测细胞活力。icartin抑制哮喘小鼠AHR,减轻嗜酸性粒细胞浸润和粘液高分泌,显著降低ova特异性细胞因子水平。此外,淫羊藿苷抑制哮喘小鼠肺组织TLR4表达,降低i -κB α磷酸化,降低NF-κB p65活化。进一步的机制研究表明,Icaritin可降低BEAS-2B细胞中tlr4诱导的炎症因子表达,阻断tlr4激活的NF-κB通路。这些发现首次表明,icartin通过抑制TLR4/NF-κB通路抑制哮喘气道炎症,提示其作为哮喘治疗药物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.20
自引率
0.00%
发文量
41
审稿时长
42 days
期刊介绍:
Pulmonary Pharmacology and Therapeutics (formerly Pulmonary Pharmacology) is concerned with lung pharmacology from molecular to clinical aspects. The subject matter encompasses the major diseases of the lung including asthma, cystic fibrosis, pulmonary circulation, ARDS, carcinoma, bronchitis, emphysema and drug delivery. Laboratory and clinical research on man and animals will be considered including studies related to chemotherapy of cancer, tuberculosis and infection. In addition to original research papers the journal will include review articles and book reviews.
Research Areas Include:
• All major diseases of the lung
• Physiology
• Pathology
• Drug delivery
• Metabolism
• Pulmonary Toxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


