Synthesis and biological evaluation of N-acyl diaryl pyrimidines (NDAPYs) as novel reverse transcriptase inhibitors
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Diaryl pyrimidines (DAPYs) are privileged structures for Non-Nucleosidic Reverse Transcriptase Inhibitors (NNRTIs), and they are considered as one of the fundamental scaffolds of existing anti-HIV agents. In this study, we designed novel molecules by the addition of a N-acyl group to the C4-position of Rilpivirine/Etravirine basic scaffold, a well-known class of DAPYs, in order to provide more interactions with K103 and E138 in Reverse Transcriptase. This was leading, as expected, to better in vitro RT inhibition, and the compounds 12c (56 %) and 12 k (49 %) demonstrated three-fold higher potency than Doravirine (17 %). Additionally, compound 12c was identified as a strong and 12k as a moderate HIV reverse transcriptase enzyme inhibitor in the in vitro experiments. DAPYs are also crucial in the development of novel anti-cancer agents targeting several kinases, and our compounds, 12d and 12j, exhibited moderate cytotoxicity with IC50 values of 14.21 and 10.16 μM, respectively, against PC-3 cells and caused cell cycle arrest at G2/M checkpoint. The compound 12d induced apoptosis and reduced angiogenesis in PC-3 cells. Additionally, preliminary kinase screening against a panel of eight human kinases demonstrated only low PIM1 inhibition. However, the absence of correlation with cytotoxicity advocates an alternative mode of action that ought to be determined. Altogether, these studies indicate that the C4-position of diaryl pyrimidines could be important and warrants further investigated towards optimization of potential reverse transcriptase inhibitors.
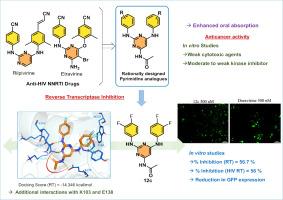
新型逆转录酶抑制剂n -酰基二芳基嘧啶的合成及生物学评价
二芳基嘧啶(DAPYs)是非核苷类逆转录酶抑制剂(NNRTIs)的特殊结构,是现有抗hiv药物的基本支架之一。在这项研究中,我们设计了一种新的分子,通过在著名的DAPYs类Rilpivirine/Etravirine基本支架的c4位置添加一个n -酰基,以提供更多与逆转录酶中的K103和E138的相互作用。正如预期的那样,这导致了更好的体外RT抑制,化合物12c(56%)和12k(49%)的效力比Doravirine(17%)高3倍。此外,化合物12c在体外实验中被鉴定为强HIV逆转录酶抑制剂,12k被鉴定为中等HIV逆转录酶抑制剂。DAPYs在针对多种激酶的新型抗癌药物的开发中也至关重要,我们的化合物12d和12j对PC-3细胞表现出中等的细胞毒性,IC50值分别为14.21和10.16 μM,并在G2/M检查点引起细胞周期阻滞。化合物12d诱导PC-3细胞凋亡,减少血管生成。此外,针对8种人类激酶的初步激酶筛选显示,PIM1仅具有低抑制作用。然而,缺乏与细胞毒性的相关性提倡一种应该确定的替代作用模式。总之,这些研究表明,二芳基嘧啶的c4位置可能是重要的,值得进一步研究以优化潜在的逆转录酶抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


