Role of Zn(II) in the aggregation of the 6aJL2R24G protein: Experimental and theoretical approach
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
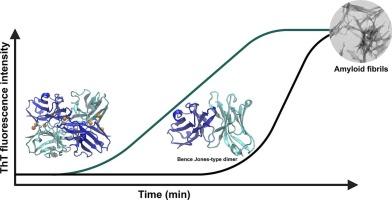
Light chain amyloidosis is a conformational disease, and one of the most common systemic amyloidosis. It is characterized by the deposition of amyloid aggregates of immunoglobulin light chains in organs and tissues. 6aJL2R24G is a recombinant variant of the λ6a germline protein, a germline present in 25 % of the amyloid-associated λ6a light chain amyloidosis cases. In this study, using spectroscopic and computational methodologies, we found that the interaction of this protein with Zn(II) accelerates amyloid fibril initiation and slows down elongation, without altering the fiber morphology. Also, neither the thermal stability nor the secondary structure are altered by the interaction with the metal ion, as measured by circular dichroism. Isothermal calorimetry titration showed that the protein has two binding sites with affinities in the micromolar range. Molecular dynamics simulations suggest that the interaction between 6aJL2R24G and Zn(II) destabilizes strand A, strand G and the EF loop, making the protein more prone to initiate the formation of amyloid fibers. Furthermore, models of 6aJL2R24G dimers and tetramers with Zn(II) suggest that Zn(II) promotes the association of the proteins, involving histidine, aspartate, and glutamate residues, with multiple different geometries, effectively raising the local protein concentration and promoting seed formation, but adopting conformations different from the one required for further monomer addition to the fiber.
Zn(II)在6aJL2R24G蛋白聚集中的作用:实验和理论方法
轻链淀粉样变性是一种构象性疾病,是最常见的系统性淀粉样变性之一。它的特点是免疫球蛋白轻链淀粉样聚集体沉积在器官和组织中。6aJL2R24G是λ6a种系蛋白的重组变体,该种系蛋白存在于25%的淀粉样蛋白相关的λ6a轻链淀粉样病病例中。在这项研究中,使用光谱和计算方法,我们发现该蛋白与Zn(II)的相互作用加速了淀粉样蛋白纤维的起始并减缓了延伸,而没有改变纤维的形态。此外,通过圆二色性测量,与金属离子的相互作用既不改变热稳定性,也不改变二级结构。等温量热滴定法表明,该蛋白具有两个亲和力在微摩尔范围内的结合位点。分子动力学模拟表明,6aJL2R24G和Zn(II)之间的相互作用使A链、G链和EF环不稳定,使蛋白质更容易启动淀粉样纤维的形成。此外,6aJL2R24G二聚体和四聚体与Zn(II)的模型表明,Zn(II)促进了蛋白质(包括组氨酸、天冬氨酸和谷氨酸残基)以多种不同的几何形状结合,有效地提高了局部蛋白质浓度并促进了种子的形成,但其构象与进一步向纤维中添加单体所需的构象不同。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


