Impaired ciliogenesis in small airway epithelial cells involves in the development of idiopathic pulmonary fibrosis
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-07-23
DOI:10.1016/j.bbadis.2025.167992
引用次数: 0
Abstract
Accumulating evidence suggests that abnormalities in airway epithelial cells involve in the development of idiopathic pulmonary fibrosis (IPF). However, whether ciliary impairment contributes to IPF pathogenesis is unspecified. In this study, we evaluated the ciliogenesis potency of IPF-derived small airway epithelial cells (SAECs), assessed the effect of aberrant ciliogenesis on lung fibroblast activation and further identified whether improving ciliogenesis could attenuate pulmonary fibrosis. Here, we showed that upon external injury or serial cell passage, IPF-derived SAECs had greater decline in ciliogenesis potency as compared to healthy control (HC). Conditioned medium harvested from SAECs post injury, serial passage or silencing the ciliogenesis regulator FOXJ1 promoted the expression of α-smooth muscle actin (α-SMA) and fibronectin (FN) in human lung fibroblasts as compared to their corresponding controls. Mice with bleomycin (BLM)-induced lung fibrosis had reduced number of ciliated cells as compared to the saline control, while overexpressing Foxj1 in mouse lung attenuated the extent of pulmonary fibrosis. LY450139, a γ-secretase inhibitor, could also improve ciliogenesis in IPF-derived SAECs and inhibit lung fibroblast activation induced by ciliogenesis impairment. We demonstrated the contributing role of ciliogenesis impairment in IPF pathogenesis. Targeting the ciliogenesis abnormality may be a potential therapeutic strategy for IPF.
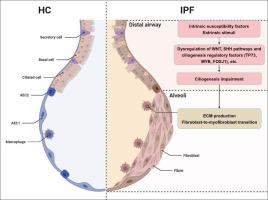
小气道上皮细胞纤毛发生受损参与特发性肺纤维化的发展。
越来越多的证据表明,气道上皮细胞异常参与特发性肺纤维化(IPF)的发展。然而,纤毛损伤是否与IPF发病机制有关尚不清楚。在这项研究中,我们评估了ipf来源的小气道上皮细胞(SAECs)的纤毛发生能力,评估了异常纤毛发生对肺成纤维细胞活化的影响,并进一步确定改善纤毛发生是否可以减轻肺纤维化。在这里,我们发现在外部损伤或连续细胞传代后,与健康对照(HC)相比,ipf衍生的saec的纤毛发生能力有更大的下降。从saec损伤后获得的条件培养基中,连续传递或沉默纤毛发生调节因子FOXJ1,与相应的对照组相比,可促进人肺成纤维细胞α-平滑肌肌动蛋白(α-SMA)和纤维连接蛋白(FN)的表达。与生理盐水对照组相比,博来霉素(BLM)诱导的肺纤维化小鼠的纤毛细胞数量减少,而小鼠肺中Foxj1的过表达减轻了肺纤维化的程度。γ-分泌酶抑制剂LY450139还能促进ipf源性saec的纤毛发生,抑制纤毛发生损伤诱导的肺成纤维细胞活化。我们证明了纤毛发生损伤在IPF发病机制中的作用。针对纤毛发生异常可能是一种潜在的治疗IPF的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


