Breaking immune evasion in breast cancer by targeting COX-2/PGE2 pathway
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
The cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway plays a pivotal role in breast cancer (BC) progression by promoting immune suppression, tumor growth, and metastasis. PGE2 mediates these effects through EP receptors (EP1–EP4), suppressing anti-tumor immunity while fostering an immunosuppressive tumor microenvironment (TME). This includes the recruitment and activation of tumor-associated macrophages (TAMs), dendritic cells (DCs), cancer-associated fibroblasts (CAFs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs), ultimately impairing cytotoxic T lymphocyte and natural killer (NK) cell function. Targeting the COX-2/PGE2 axis presents a promising strategy for BC treatment. Dual inhibition of EP2 and EP4 has demonstrated superior efficacy in reversing immune suppression compared to single-receptor blockade. Additionally, combining EP4 antagonists with immune checkpoint inhibitors (ICIs) such as anti-PD-1 and anti-CTLA-4 enhances T cell infiltration and tumoricidal activity, leading to improved therapeutic outcomes. Another emerging approach involves enhancing the activity of 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the key enzyme responsible for PGE2 degradation, to counteract PGE2-driven immune evasion. PTGES1 inhibitors have shown great potential in overcoming the immunosuppressive TME in BC patients. Elevated TIL levels in TNBC and HER2-positive BC are associated with improved prognosis; however, COX-2 inhibitors such as celecoxib failed to enhance survival and carry potential cardiovascular risks, highlighting the need for TIL-stratified trials to refine immunotherapeutic strategies. This review highlights the immunosuppressive mechanisms of the COX-2/PGE2 pathway in BC and explores novel therapeutic strategies targeting this axis. Understanding the intricate crosstalk between PGE2 signaling and immune modulation may lead to the development of more effective BC treatments, particularly in combination with immunotherapies.
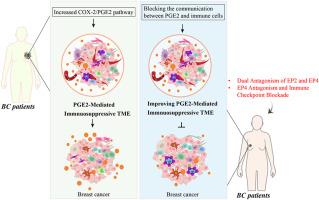
靶向COX-2/PGE2通路打破乳腺癌免疫逃避
环氧化酶-2 (COX-2)/前列腺素E2 (PGE2)通路通过促进免疫抑制、肿瘤生长和转移在乳腺癌(BC)进展中起关键作用。PGE2通过EP受体(EP1-EP4)介导这些作用,抑制抗肿瘤免疫,同时培养免疫抑制肿瘤微环境(TME)。这包括肿瘤相关巨噬细胞(tam)、树突状细胞(DCs)、癌症相关成纤维细胞(CAFs)、髓源性抑制细胞(MDSCs)和调节性T细胞(Tregs)的募集和激活,最终损害细胞毒性T淋巴细胞和自然杀伤细胞(NK)功能。针对COX-2/PGE2轴是一种很有前景的BC治疗策略。与单受体阻断相比,双重抑制EP2和EP4在逆转免疫抑制方面显示出更好的效果。此外,EP4拮抗剂与免疫检查点抑制剂(ICIs)如抗pd -1和抗ctla -4联合使用可增强T细胞浸润和肿瘤杀伤活性,从而改善治疗效果。另一种新出现的方法涉及提高15-羟基前列腺素脱氢酶(15-PGDH)的活性,这是负责PGE2降解的关键酶,以抵消PGE2驱动的免疫逃避。PTGES1抑制剂在克服BC患者的免疫抑制性TME方面显示出巨大的潜力。TNBC和her2阳性BC中TIL水平升高与预后改善相关;然而,COX-2抑制剂如塞来昔布未能提高生存率,并携带潜在的心血管风险,这突出了对til分层试验的需求,以完善免疫治疗策略。本文综述了BC中COX-2/PGE2通路的免疫抑制机制,并探索了针对该轴的新治疗策略。了解PGE2信号和免疫调节之间复杂的串扰可能会导致更有效的BC治疗的发展,特别是与免疫疗法的结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


