Real-world outcomes of Adjuvant De Gramont versus Xelox chemotherapy in reSected gasTric cancER: a propensity score-matched analysis (ASTER study)
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
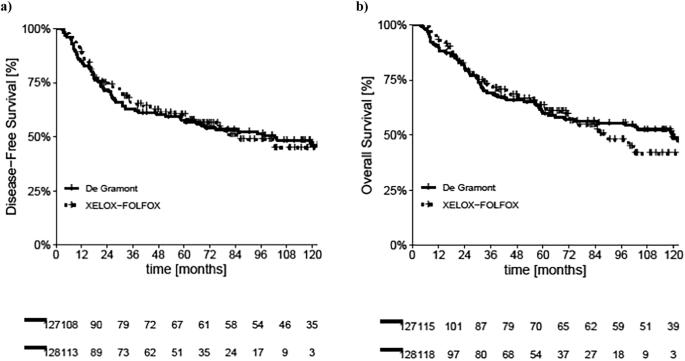
The role of adjuvant chemotherapy (aCT) in gastric and esophago-gastric junction cancer (GC/EGJC) remains controversial. This study (ASTER study) aimed to compare the clinical outcomes of De Gramont (DG) versus XELOX/FOLFOX (OXA) regimens in a European real-world setting. This retrospective, bicentric study included patients treated with aCT between January 2001 and January 2018. A propensity score-matched (PSM) analysis was performed to compare oncological outcomes between DG and OXA regimens. Primary endpoints were disease-free survival (DFS) and overall survival (OS). Statistical analyses included the chi-square test, Kaplan–Meier method, and Cox proportional hazards modeling. Among 255 patients (127 DG, 128 OXA), 160 were matched (80 per arm) by PSM. Median DFS and OS did not differ significantly between groups (mDFS: 102.3 vs. 85.4 months, p = 0.91; mOS: 119.5 vs. 89.8 months, p = 0.69). In PSM-adjusted analysis, DG showed a trend towards longer DFS (p = 0.052) and significantly improved OS (p = 0.016). Multivariate analysis confirmed age, ECOG PS, resection margins, and stage as major prognostic factors. DG and OXA regimens demonstrated similar efficacy in the adjuvant treatment of resected GC/GEJC in a European cohort. Further prospective studies are warranted to optimize regimen selection and refine patient stratification.
辅助德格拉蒙与Xelox化疗在切除胃癌中的实际结果:倾向评分匹配分析(ASTER研究)。
辅助化疗(aCT)在胃癌和食管胃结癌(GC/EGJC)中的作用仍存在争议。本研究(ASTER研究)旨在比较欧洲现实环境中De Gramont (DG)与XELOX/FOLFOX (OXA)方案的临床结果。这项回顾性、双中心研究纳入了2001年1月至2018年1月期间接受aCT治疗的患者。进行倾向评分匹配(PSM)分析,比较DG和OXA方案之间的肿瘤结果。主要终点为无病生存期(DFS)和总生存期(OS)。统计分析包括卡方检验、Kaplan-Meier法和Cox比例风险模型。在255例患者中(127例DG, 128例OXA), 160例(每组80例)通过PSM配对。两组间中位DFS和OS无显著差异(mDFS: 102.3个月vs 85.4个月,p = 0.91;生存期:119.5 vs. 89.8个月,p = 0.69)。在psm校正分析中,DG表现出延长DFS (p = 0.052)和显著改善OS (p = 0.016)的趋势。多因素分析证实年龄、ECOG PS、切除边缘和分期是主要的预后因素。在一项欧洲队列研究中,DG和OXA方案在切除的GC/GEJC的辅助治疗中显示出相似的疗效。需要进一步的前瞻性研究来优化方案选择和细化患者分层。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


