YY1-glycolytic axis promotes high glucose-induced cancer stemness in endometrial cancer: A multi-omics guided therapeutic strategy
IF 10.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Abnormal glucose metabolism in patients with diabetes mellitus or hyperglycaemia is a key factor leading to poor prognosis of endometrial cancer (EC) and warrants further exploration of its underlying mechanisms. In this study, we employed an integrative multiomics approach, including proteomics, transcriptomics, nontargeted metabolomics and single-cell metabolomics, and revealed that high glucose promotes EC cell stemness, thereby promoting tumour progression and reducing the sensitivity of EC cells to cisplatin (platinum-PtII). Mechanistically, there is a significant association between enhanced stemness and increased glycolytic activity in EC cells, and the transcription factor YY1 was found to be a key regulator of PDK1, CD133, and CD44 under high-glucose conditions, with YY1 binding to their promoter regions. Inhibiting YY1 expression effectively attenuates the stem cell properties of tumour cells and increases their sensitivity to cisplatin. Furthermore, we developed ROS-responsive nanoparticles for the codelivery of C8-PtIV-COOH and YY1 siRNA (NP-PtIV/siYY1), which synergistically amplify antitumour effects and chemosensitivity in patient-derived xenograft (PDX)-bearing mice with diabetes. Taken together, our results demonstrated that YY1 is a promising therapeutic target for inhibiting EC stemness and overcoming chemoresistance, particularly under high-glucose conditions.
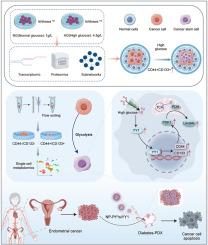
yy1 -糖酵解轴促进高糖诱导的子宫内膜癌癌干性:一种多组学指导的治疗策略
糖尿病或高血糖患者糖代谢异常是导致子宫内膜癌(EC)预后不良的关键因素,其机制值得进一步探讨。在这项研究中,我们采用了综合多组学方法,包括蛋白质组学、转录组学、非靶向代谢组学和单细胞代谢组学,发现高糖可促进EC细胞的干细胞性,从而促进肿瘤进展,降低EC细胞对顺铂(铂- ptii)的敏感性。在机制上,EC细胞的干性增强与糖酵解活性增加之间存在显著关联,并且发现转录因子YY1是高糖条件下PDK1、CD133和CD44的关键调节因子,YY1与它们的启动子区域结合。抑制YY1表达可有效减弱肿瘤细胞的干细胞特性,增加肿瘤细胞对顺铂的敏感性。此外,我们开发了ros响应纳米颗粒,用于共同递送C8-PtIV-COOH和YY1 siRNA (NP-PtIV/siYY1),这可以协同增强患者源性异种移植(PDX)携带糖尿病小鼠的抗肿瘤作用和化疗敏感性。综上所述,我们的研究结果表明YY1是抑制EC干细胞和克服化疗耐药的有希望的治疗靶点,特别是在高糖条件下。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


