Hyperactivation of Transforming Growth Factor-β–Activated Kinase 1 Causes Skeletal Muscle Pathology Reminiscent of Inflammatory Myopathies
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
Abstract
Loss of skeletal muscle mass and strength is a debilitating consequence of various chronic diseases, inflammatory myopathies, and neuromuscular disorders. Inflammation plays a major role in the perpetuation of myopathy in degenerative muscle diseases. Transforming growth factor-β–activated kinase 1 (TAK1) is a major signaling protein that mediates the activation of multiple intracellular signaling pathways in response to inflammatory cytokines and microbial products. Recent studies have demonstrated that TAK1 is essential for the growth and maintenance of skeletal muscle mass in adult mice. However, the effects of overstimulation of TAK1 activity in the regulation of skeletal muscle mass remain unknown. The present study investigated the effects of varying levels of TAK1 activation on skeletal muscle in adult mice. Results showed that although low levels of TAK1 activation improve skeletal muscle mass, sustained hyperactivation of TAK1 causes myopathy in adult mice. Excessive stimulation of TAK1 manifested pathologic features, such as myofiber degeneration and regeneration, cellular infiltration, increased expression of proinflammatory molecules, and interstitial fibrosis. Hyperactivation of TAK1 also up-regulated proteolytic systems and various catabolic signaling pathways in skeletal muscle of adult mice. Altogether, this study demonstrated that physiological levels of activation of TAK1 lead to myofiber hypertrophy, whereas its hyperactivation results in myofiber damage and other pathologic features resembling inflammatory myopathies.
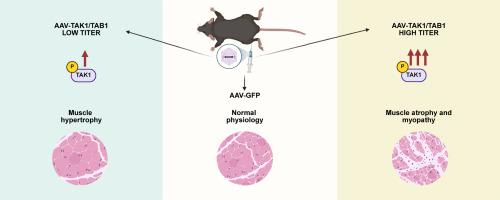
TAK1的过度激活引起骨骼肌病理,使人联想到炎症性肌病。
骨骼肌质量和力量的丧失是各种慢性疾病、炎症性肌病和神经肌肉疾病的衰弱后果。在退行性肌肉疾病中,炎症在肌病的延续中起着重要作用。TGF-β-活化激酶1 (TAK1)是一种主要的信号蛋白,在炎症细胞因子和微生物产物的反应中介导多种信号通路的激活。最近的研究表明,TAK1对于成年小鼠骨骼肌质量的生长和维持至关重要。然而,过度刺激TAK1活性在骨骼肌质量调节中的作用尚不清楚。本研究探讨了不同水平的TAK1激活对成年小鼠骨骼肌的影响。结果表明,虽然低水平的TAK1激活可以改善骨骼肌质量,但在成年小鼠中,TAK1的持续过度激活会导致肌病。过度刺激TAK1表现为肌纤维变性再生、细胞浸润、促炎分子表达增加、间质纤维化等病理特征。TAK1的过度激活也上调了成年小鼠骨骼肌的蛋白水解系统和各种分解代谢信号通路。总之,本研究表明生理水平的TAK1激活导致肌纤维肥大,而其过度激活导致肌纤维损伤和其他类似炎症性肌病的病理特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


