High glutamate levels in the bone marrow of multiple myeloma patients promote osteoclast formation: a novel target for osteolytic bone disease
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
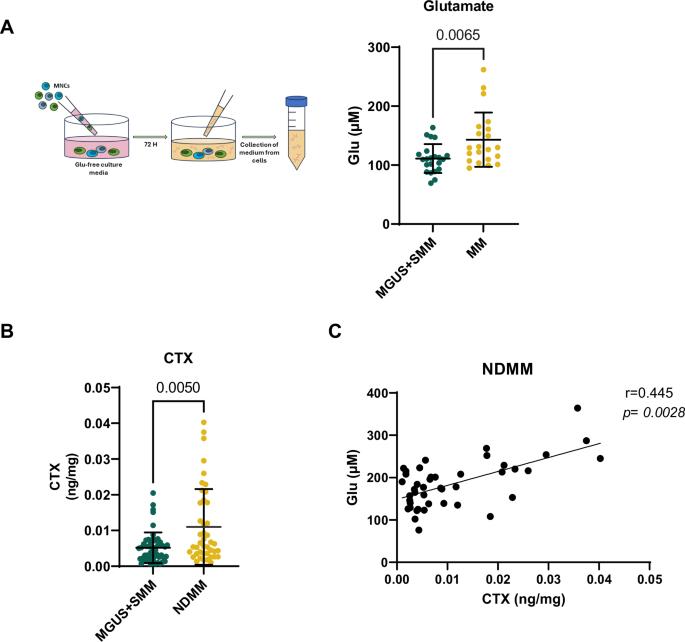
Multiple Myeloma (MM) is a glutamine (Gln)-addicted cancer. Consequently, the MM bone microenvironment (BM) is characterized by lower Gln and higher glutamate (Glu) levels than those in pre-malignant monoclonal gammopathies. Such MM-dependent metabolic perturbation impairs osteoblast differentiation in the bone microenvironment, but its effect on osteoclast (OCL) bone resorption is still unknown. We first show that bone marrow mononuclear cells from MM patients release higher levels of Glu compared to those from patients with monoclonal gammopathy of undetermined significance (MGUS) or smoldering multiple myeloma (SMM). This increased Glu production correlates with elevated bone resorption activity. We then demonstrate that Glu stimulates OCL differentiation via the activation of NF-κB-NFATc1 pathway in low-Glu BM samples from pre-malignant patients but not in high-Glu samples of MM patients. Secondly, the early phase of OCL formation was associated with high Glu intracellular content and induction of the Glu transporter EAAT1. Consistently, the pharmacological inhibition of EAAT1 hinders OCL differentiation by blocking the RANKL-dependent signaling pathway and actin cytoskeleton reorganization. Overall, our data indicate that high Glu levels in MM bone marrow are involved in OCL formation, suggesting that targeting Glu transport may represent a novel approach for the prevention of osteolytic lesions in MM patients.
多发性骨髓瘤患者骨髓中的高谷氨酸水平促进破骨细胞形成:溶骨性骨病的新靶点
多发性骨髓瘤(MM)是一种谷氨酰胺(Gln)成瘾的癌症。因此,MM骨微环境(BM)的特点是Gln水平较低,谷氨酸(Glu)水平高于恶性前单克隆伽玛病。这种mm依赖性代谢扰动会损害骨微环境中成骨细胞的分化,但其对破骨细胞(OCL)骨吸收的影响尚不清楚。我们首先表明,与未确定意义的单克隆γ病(MGUS)或阴生多发性骨髓瘤(SMM)患者相比,MM患者的骨髓单核细胞释放的Glu水平更高。这种增加的谷氨酸产生与骨吸收活性升高有关。我们随后证明,Glu通过激活NF-κB-NFATc1途径,在恶性前患者的低Glu BM样本中刺激OCL分化,而在MM患者的高Glu样本中则没有。其次,OCL形成的早期阶段与细胞内高谷氨酸含量和谷氨酸转运体EAAT1的诱导有关。一致地,EAAT1的药理抑制通过阻断rankl依赖的信号通路和肌动蛋白细胞骨架重组来阻碍OCL分化。总的来说,我们的数据表明,MM骨髓中高水平的Glu参与了OCL的形成,这表明靶向Glu运输可能是预防MM患者溶骨性病变的一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


