Pharmacogenetics association with long-term clinical evolution in a kidney transplant patients cohort
Q2 Agricultural and Biological Sciences
Current Research in Pharmacology and Drug Discovery
Pub Date : 2025-01-01
DOI:10.1016/j.crphar.2025.100230
引用次数: 0
Abstract
Background
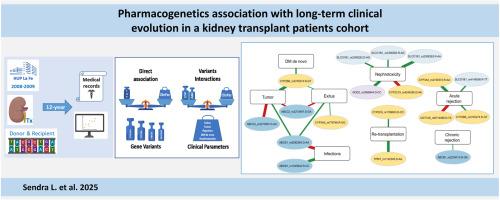
Pharmacogenetic variability has been reported to influence the efficacy and safety of immunosuppressive therapies in early stages of kidney transplantation. This study investigates long-term associations between pharmacogene variants and clinical outcomes in a cohort of kidney transplant recipients over a 12-year follow-up.
Materials and methods
We analyzed 37 SNPs from 14 genes related to drug metabolism and transport in 79 kidney transplant patients. Clinical parameters, including survival, renal function, tumor occurrence, and pharmacokinetics of tacrolimus, were evaluated. Logistic regression and Kaplan-Meier analyses assessed associations between gene variants and clinical outcomes.
Results
Variants in metabolizer (CYP3A5, CYP2B6) and transporter genes (ABCB1, ABCC2) were associated with 12-year survival. Increased tumor risk correlated with ABCC2 variants in donors and decreased risk with CYP2B6 rs3745274 in recipients. Renal function was influenced by variants in ABCB1, ABCC2, CYP3A5, CYP3A4, and CYP2B6. Tacrolimus dose-dependent concentration was affected by variants in CYP3A4, CYP3A5, CYP2C19, ABCB1, and SLCO1B1. Increased nephrotoxicity risk was associated with CYP2C19 rs4244285 and reduced by SLCO1B1 rs2306283 AA and AG variants. Gene variant interactions between metabolizer and transporter genes were also associated with altered risk of events incidence.
Discussion
Our findings support that pharmacogene variants influence transplant outcomes. Notable associations include survival related to ABCB1 and ABCC2 variants, tumor occurrence linked to CYP2B6 rs3745274, and renal function affected by multiple pharmacogenes. Variants in CYP2C19 and SLCO1B1 significantly impacted tacrolimus pharmacokinetics and nephrotoxicity risk. These results underline the importance of pharmacogenetic testing for personalized management in kidney transplantation, although further validation in larger cohorts is necessary.
药物遗传学与肾移植患者长期临床演变的关系
已有报道称药物遗传变异会影响肾移植早期免疫抑制治疗的有效性和安全性。本研究对一组肾移植受者进行了为期12年的随访,调查了药物基因变异与临床结果之间的长期关系。材料与方法对79例肾移植患者的14个药物代谢和转运相关基因的37个snp进行分析。临床参数包括生存、肾功能、肿瘤发生和他克莫司的药代动力学。逻辑回归和Kaplan-Meier分析评估了基因变异与临床结果之间的关系。结果代谢基因(CYP3A5、CYP2B6)和转运基因(ABCB1、ABCC2)变异与12年生存率相关。供体中ABCC2变异与肿瘤风险增加相关,受体中CYP2B6 rs3745274变异与肿瘤风险降低相关。肾功能受ABCB1、ABCC2、CYP3A5、CYP3A4和CYP2B6变异的影响。他克莫司剂量依赖性浓度受CYP3A4、CYP3A5、CYP2C19、ABCB1和SLCO1B1基因变异的影响。增加的肾毒性风险与CYP2C19 rs4244285相关,与SLCO1B1 rs2306283 AA和AG变体相关。代谢基因和转运基因之间的基因变异相互作用也与事件发生风险的改变有关。我们的研究结果支持药物基因变异影响移植结果。值得注意的关联包括与ABCB1和ABCC2变异相关的生存,与CYP2B6 rs3745274相关的肿瘤发生,以及受多种药物基因影响的肾功能。CYP2C19和SLCO1B1的变异显著影响他克莫司的药代动力学和肾毒性风险。这些结果强调了药物遗传学检测对肾移植个体化管理的重要性,尽管需要在更大的队列中进一步验证。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Pharmacology and Drug Discovery
Agricultural and Biological Sciences-Animal Science and Zoology
CiteScore
6.40
自引率
0.00%
发文量
65
审稿时长
40 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


