Anti-cancer effect of interleukin-2 fused to flagellin expressed by tumor-targeting Salmonella
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Interleukin-2 (IL2) treatment has been explored as a potent immunotherapy agent, particularly for cancers, due to its ability to stimulate T cell proliferation and activity. However, significant challenges and limitations are associated with IL2 treatment, including its short half-life, systemic toxicity and side effects, and limited efficacy in solid tumors. In this study, we deployed an attenuated Salmonella Gallinarum (SG), an avian-specific pathogen capable of targeting tumor tissue, to express and secrete the IL2 using a bacterial flagellum type 3 secretion system (T3SS). Since the T3SS is used for the secretion of flagellin monomers (FliC), DNA of the human IL2 gene was fused to the SG fliC gene so that the fusion proteins would be exported together. A superb anti-cancer effect was observed when the SG expressing and secreting the FliC-IL2 fusion protein was injected into a syngeneic tumor mouse model with CT26 colorectal cancer via the tail vein. Within the fusion protein, the FliC moiety led to a selective increase in MHCIIhighCD206- M1-like macrophages, while the IL2 moiety promoted selective expansion of cytotoxic CD8+ T cells and NK cells, without expanding CD4+FoxP3+ regulatory T cells in the tumor microenvironment (TME). It was concluded that the local delivery of IL2 within the TME by cancer-targeting SG could overcome the limitations associated with IL2-based cancer immunotherapy.
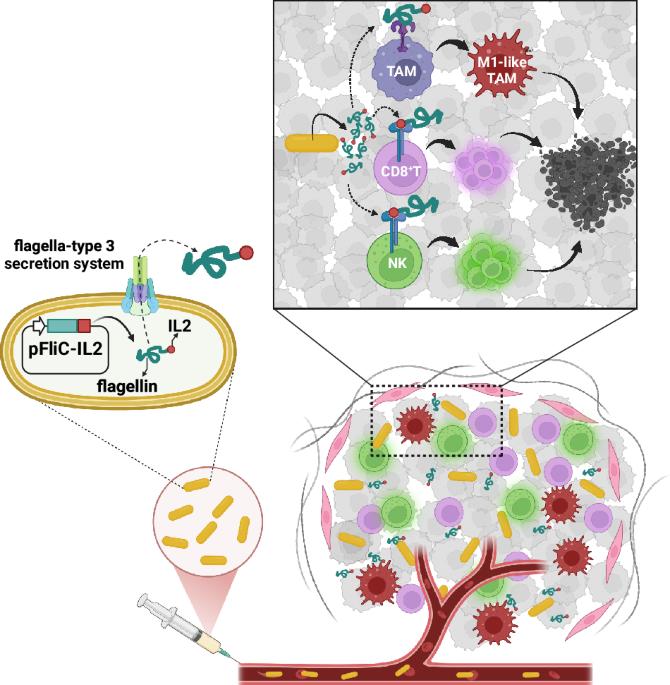
肿瘤靶向沙门氏菌表达的白细胞介素-2与鞭毛蛋白融合的抗癌作用。
由于白细胞介素-2 (IL2)能够刺激T细胞的增殖和活性,因此已被探索为一种有效的免疫治疗药物,特别是对于癌症。然而,与il - 2治疗相关的重大挑战和局限性,包括其半衰期短,全身毒性和副作用,以及对实体肿瘤的疗效有限。在这项研究中,我们利用一种减毒鸡沙门氏菌(SG),一种能够靶向肿瘤组织的家禽特异性病原体,通过细菌鞭毛3型分泌系统(T3SS)表达和分泌il - 2。由于T3SS用于鞭毛蛋白单体(FliC)的分泌,因此将人il - 2基因的DNA与SG FliC基因融合,使融合蛋白一起输出。将表达和分泌FliC-IL2融合蛋白的SG经尾静脉注射到CT26结直肠癌的同基因肿瘤小鼠模型中,观察到极好的抗癌效果。在融合蛋白中,FliC片段导致MHCIIhighCD206- m1样巨噬细胞的选择性增加,而IL2片段促进细胞毒性CD8+ T细胞和NK细胞的选择性扩增,而不扩增肿瘤微环境(TME)中的CD4+FoxP3+调节性T细胞。由此可见,靶向肿瘤的SG在TME内局部递送il - 2可以克服基于il - 2的癌症免疫治疗的局限性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


