Pyroptosis-Mediated Antitumor Activity of Cinobufagin in Non-Small Cell Lung Cancer
Abstract
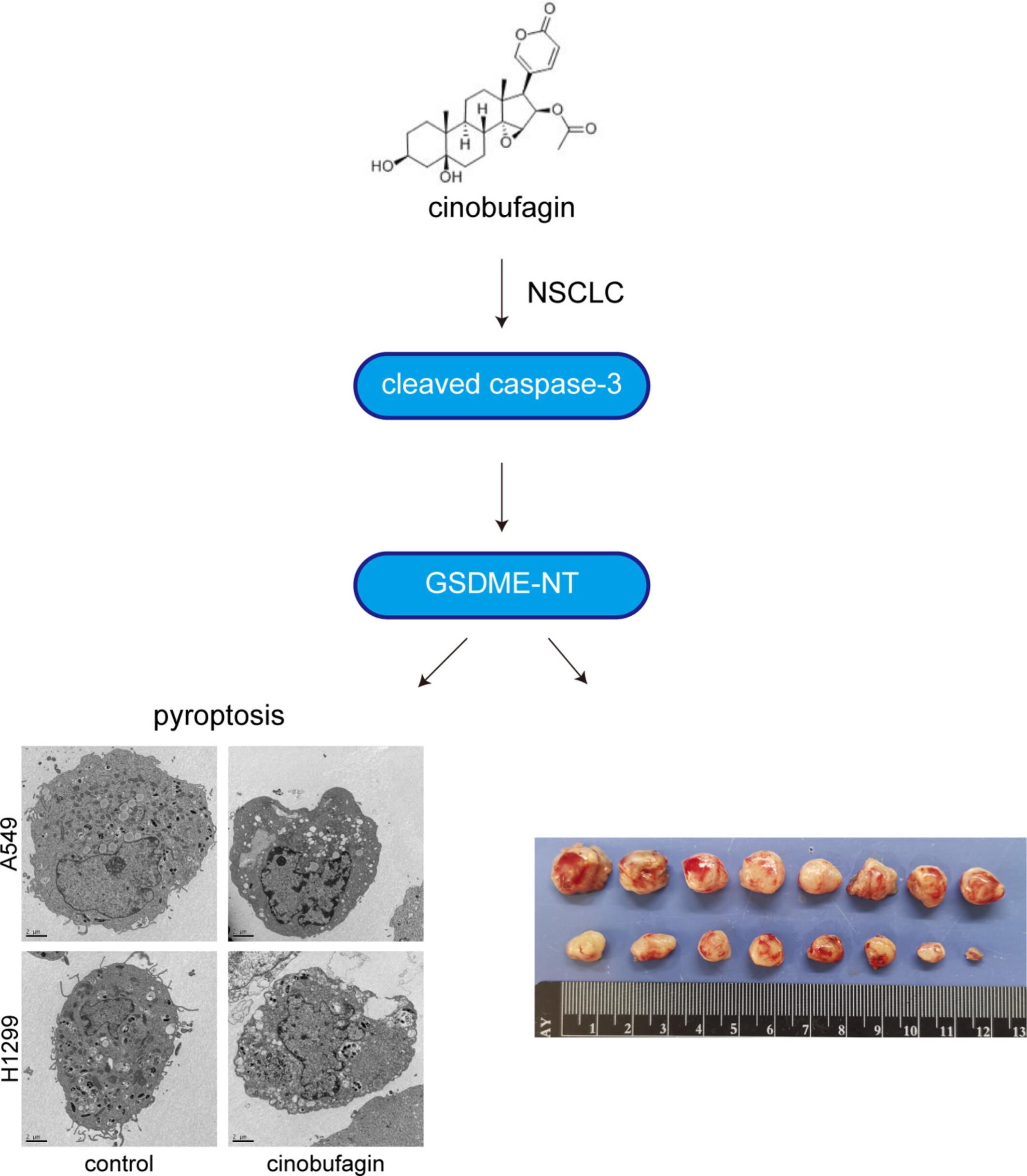
This study aims to investigate the therapeutic efficacy and molecular mechanism of cinobufagin in non-small cell lung cancer (NSCLC) via pyroptosis induction. Bronchial epithelial cells and NSCLC cell lines were treated with gradient concentrations of cinobufagin. Cell viability was evaluated using Cell Counting Kit-8 (CCK-8) assay. RNA-sequencing was performed to identify differentially expressed genes. Lactate dehydrogenase (LDH) release was measured via cytotoxicity detection kit. Pyroptotic morphological changes were observed by transmission electron microscopy. Western blotting analysed expression levels of pyroptosis-related proteins. In vivo efficacy was validated in nude mouse xenograft models. Immunohistochemistry evaluated tumour pyroptosis markers, whilst flow cytometry analysed tumour-infiltrating CD8+ T cells and natural killer (NK) cells. Cinobufagin demonstrated selective cytotoxicity against NSCLC cells with minimal toxicity to normal bronchial epithelium. RNA-seq analysis revealed significant enrichment of pyroptosis-related pathways. Functional experiments confirmed cinobufagin-induced LDH release, characteristic pyroptotic morphological changes and upregulation of cleaved caspase-3 and Gasdermin E (GSDME)-NT in NSCLC cells. In xenograft models, cinobufagin treatment reduced tumour volume compared to controls. Mechanistically, this was associated with enhanced caspase-3 activation and GSDME-NT accumulation in tumour tissues. Notably, cinobufagin treatment significantly increased NK cell infiltration and activity. Cinobufagin exerts antitumor effects in NSCLC through caspase-3/GSDME-mediated pyroptosis induction, accompanied by immune microenvironment modulation. These findings provide preclinical evidence for cinobufagin as a potential therapeutic agent targeting pyroptosis in NSCLC.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


