The source of dietary fat influences anti-tumour immunity in obese mice
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
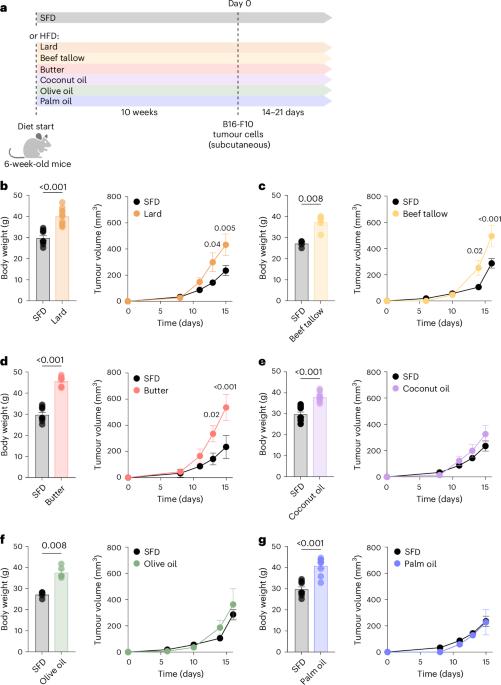
Obesity increases the risk of many cancers and impairs the anti-tumour immune response. However, little is known about whether the source or composition of dietary fat affects tumour growth or anti-tumour immunity in obesity. Here, we show that high-fat diets (HFDs) derived from lard, beef tallow or butter accelerate tumour growth in a syngeneic model of melanoma, but HFDs based on coconut oil, palm oil or olive oil do not, despite equivalent obesity. Using butter-based and palm oil-based HFDs as examples, we find that these dietary fat sources differentially regulate natural killer and CD8 T cell infiltration and function within the tumour microenvironment, governed by distinct effects on the plasma metabolome and intracellular metabolism. We identify diet-related lipid intermediates, namely long-chain acylcarnitine species, as immunosuppressive metabolites enriched in mice fed butter compared to palm oil HFD. Together, these results highlight the significance of diet in maintaining a healthy immune system and suggest that modifying dietary fat may improve cancer outcomes in obesity. This study shows that animal-based high-fat diets accelerate tumour growth and impair anti-tumour response to melanoma in obese mice, whereas plant-based high-fat diets do not.

饮食脂肪的来源影响肥胖小鼠的抗肿瘤免疫
肥胖会增加患多种癌症的风险,并损害抗肿瘤免疫反应。然而,对于饮食脂肪的来源或成分是否会影响肥胖患者的肿瘤生长或抗肿瘤免疫,人们知之甚少。在这里,我们展示了高脂肪饮食(HFDs)来自猪油,牛油或黄油加速黑色素瘤的同基因模型的肿瘤生长,但基于椰子油,棕榈油或橄榄油的HFDs没有,尽管相同的肥胖。以黄油和棕榈油为基础的HFDs为例,我们发现这些膳食脂肪来源通过对血浆代谢组和细胞内代谢的不同影响来调节肿瘤微环境中的自然杀伤细胞和CD8 T细胞的浸润和功能。我们确定了与饮食相关的脂质中间体,即长链酰基肉碱,作为免疫抑制代谢物,在喂食黄油的小鼠中比喂食棕榈油HFD的小鼠中富集。总之,这些结果强调了饮食在维持健康免疫系统中的重要性,并表明改变饮食中的脂肪可能会改善肥胖患者的癌症预后。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


