Deubiquitinase USP6 stabilizes oncogenic RUNX1 fusion proteins to promote the leukemic potential and malignant progression
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
RUNX1-rearranged leukemia is one of the most common subtypes of leukemia associated with genetic abnormalities. Although the majority of patients respond to chemotherapy, relapse and long-term adverse effects remain significant challenges. RUNX1 fusions, resulting from chromosomal rearrangements, are pivotal oncogenic drivers, with over 70 distinct variants identified. Therefore, elucidating their regulatory mechanisms may help to develop novel therapeutic strategies. Herein, we identify a universal deubiquitinase, USP6, that stabilizes RUNX1 fusion proteins with different partners. Importantly, USP6 is specifically upregulated in RUNX1-rearranged leukemia and strongly correlates with poor patient outcomes. Mechanistically, USP6 stabilizes RUNX1 fusions to facilitate the formation of phase separation, leading to robust transcriptional activation of the fusions. Depletion of USP6 dramatically inhibits proliferation and induces differentiation of RUNX1-rearranged leukemic cells. The marketed drug auranofin is identified as a potential USP6 inhibitor, which induces degradation of different RUNX1 fusions, further triggering myeloid differentiation and arresting xenograft tumor growth. Notably, auranofin exhibits selective therapeutic efficacy in patient-derived leukemia blasts from RUNX1-rearranged cases. Together, we not only uncover a new biological function of USP6 in regulating the transcriptional activity of RUNX1 fusions but also validate USP6 as a promising drug target and auranofin as a candidate therapy for RUNX1-rearranged leukemia.
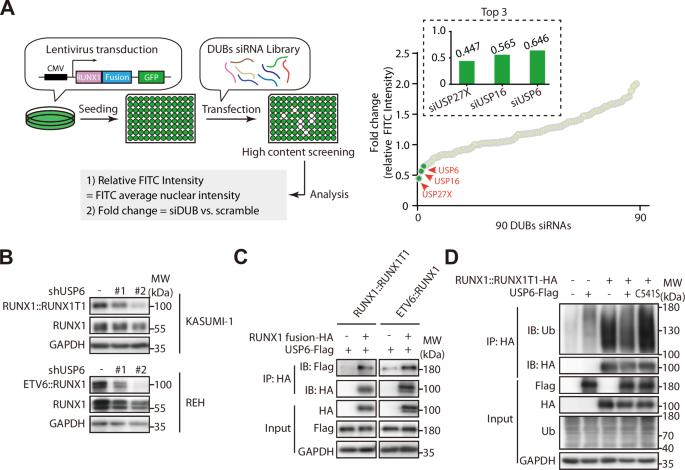
去泛素酶USP6稳定致癌RUNX1融合蛋白,促进白血病潜能和恶性进展
runx1重排白血病是与遗传异常相关的最常见白血病亚型之一。虽然大多数患者对化疗有反应,但复发和长期不良反应仍然是重大挑战。由染色体重排引起的RUNX1融合是关键的致癌驱动因素,已确定有70多种不同的变体。因此,阐明它们的调控机制可能有助于开发新的治疗策略。在这里,我们发现了一种通用的去泛素酶USP6,它可以稳定RUNX1融合蛋白与不同的伴侣。重要的是,USP6在runx1重排白血病中特异性上调,并与不良患者预后密切相关。在机制上,USP6稳定RUNX1融合以促进相分离的形成,从而导致融合的强大转录激活。USP6的缺失显著抑制runx1重排白血病细胞的增殖并诱导其分化。已上市的药物auranofin被确定为潜在的USP6抑制剂,可诱导不同RUNX1融合物的降解,进一步触发骨髓分化并阻止异种移植肿瘤的生长。值得注意的是,在runx1重排病例的患者源性白血病原细胞中,金糠蛋白显示出选择性治疗效果。总之,我们不仅发现了USP6在调节RUNX1融合体转录活性方面的新生物学功能,而且证实了USP6是一个有希望的药物靶点,金糠蛋白是RUNX1重排白血病的候选治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


