Superoxide-mediated phosphorylation and stabilization of Mcl-1 by AKT underlie venetoclax resistance in hematologic malignancies
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Resistance to the Bcl-2-specific inhibitor, Venetoclax (VEN), poses a therapeutic challenge in the management of chronic lymphocytic leukemia and acute myeloid leukemia. Although VEN resistance has been linked to Mcl-1 upregulation, thereby switching survival dependence from Bcl-2 to Mcl-1, the mechanism underlying increased Mcl-1 expression remains elusive. Given that changes in cellular redox state affect cancer cell fate, we investigated the crosstalk between intracellular redox milieu and Mcl-1 upregulation in VEN-resistant cells. Results show that increased Mcl-1 protein levels in VEN-resistant hematologic malignant cells are associated with elevated intracellular superoxide (O2.−) levels. Validating that, augmenting intracellular O2.− in VEN-sensitive cells increases Mcl-1 phosphorylation at threonine-163 (T163pMcl-1) and protein stability via reduced Mcl-1 ubiquitination and degradation. Furthermore, redox-activated AKT/PKB is implicated in O2.−-induced T163pMcl-1, as reducing intracellular O2.− or inhibiting AKT significantly decreases T163pMcl-1 and Mcl-1 accumulation, which amplifies mitochondrial apoptotic priming and restores VEN sensitivity. Importantly, combination therapy with AKT inhibitor, capivasertib, and VEN reduced VEN-resistant cells systemically and prolonged survival in a murine model. Collectively, a novel redox-dependent mechanism of Mcl-1 stability is demonstrated for the acquisition of VEN resistance, which has therapeutic implications for employing redox modulating strategies and AKT inhibitors against VEN-resistant hematologic malignancies.

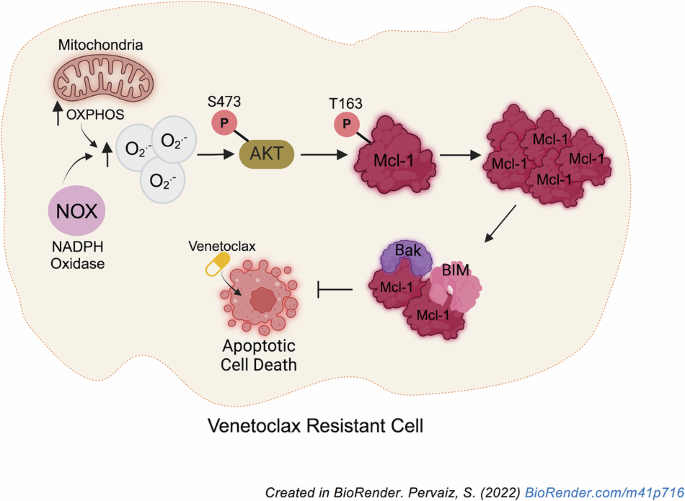
AKT介导的超氧化物介导的Mcl-1磷酸化和稳定是恶性血液病中venetoclax耐药的基础
bcl -2特异性抑制剂Venetoclax (VEN)的耐药性对慢性淋巴细胞白血病和急性髓性白血病的治疗提出了挑战。尽管VEN耐药与Mcl-1上调有关,从而将生存依赖从Bcl-2转变为Mcl-1,但Mcl-1表达增加的机制尚不清楚。考虑到细胞氧化还原状态的改变会影响癌细胞的命运,我们研究了ven耐药细胞中细胞内氧化还原环境与Mcl-1上调之间的串扰。结果表明,在ven抗性血液恶性细胞中,Mcl-1蛋白水平升高与细胞内超氧化物(O2.−)水平升高有关。证实了这一点,增加了细胞内氧气。−在vin敏感细胞中,通过降低Mcl-1泛素化和降解,增加Mcl-1在苏氨酸-163 (T163pMcl-1)的磷酸化和蛋白质稳定性。此外,氧化还原激活的AKT/PKB与O2有关。−诱导T163pMcl-1,降低细胞内O2。−或抑制AKT可显著降低T163pMcl-1和Mcl-1的积累,从而放大线粒体凋亡启动,恢复VEN敏感性。重要的是,在小鼠模型中,AKT抑制剂、capivasertib和VEN联合治疗可全身减少VEN耐药细胞并延长存活时间。综上所述,一种新的氧化还原依赖的Mcl-1稳定性机制被证明可以获得VEN抗性,这对于使用氧化还原调节策略和AKT抑制剂治疗VEN抗性血液恶性肿瘤具有治疗意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


