Predicting colorectal cancer risk in FAP patients using patient-specific organoids
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Colorectal cancer (CRC), a prevalent global cancer, is mostly sporadic. Familial adenomatous polyposis (FAP), arises from APC germline mutations. We established FAP-human embryonic stem cell lines (FAP1,2,3) with distinct APC mutations and differentiated them into colon organoids to study cancer development. While normal expressing APC lines and FAP3 formed complex organoids, FAP1,2 failed to differentiate. By utilizing CRISPR editing to correct APC mutations in FAP1,2, we succeeded in restoring their ability to form complex organoids expressing colon gene (CDX2). To elucidate the truncated APC proteins’ mechanism of action, we used AlphaFold2 algorithm to model their secondary structures. Structural analysis of the normal phenotype organoids (normal and FAP3) revealed 5-6 salt bridges only at the N-terminal oligomerization domain. In contrast, analysis of disease organoids-phenotype (FAP1,2) revealed a production of novel salt bridges, likely act in a dominant-negative manner on full-length APC, disrupting APC function and promoting tumorigenesis. Our study underscores the critical role of germline APC mutations in colon cancer initiation, revealing how specific mutations influence disease severity. By deciphering APC structure-function relationships, we illuminate potential therapies and the molecular underpinnings of APC mutations that precede clinical presentation.
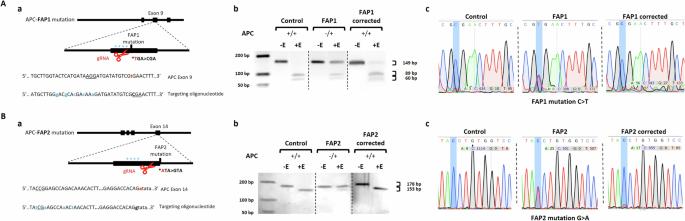
使用患者特异性类器官预测FAP患者的结直肠癌风险。
结直肠癌(CRC)是一种常见的全球癌症,大多是散发性的。家族性腺瘤性息肉病(FAP),由APC种系突变引起。我们建立了具有不同APC突变的fap -人胚胎干细胞系(FAP1、2、3),并将其分化为结肠类器官,以研究癌症的发生。正常表达的APC细胞系和FAP3形成复杂的类器官,而FAP1、2不能分化。通过利用CRISPR编辑来纠正FAP1、2中的APC突变,我们成功地恢复了它们形成表达结肠基因(CDX2)的复杂类器官的能力。为了阐明截断的APC蛋白的作用机制,我们使用AlphaFold2算法对其二级结构进行建模。对正常表型类器官(正常型和FAP3型)的结构分析显示,仅在n端寡聚结构域存在5-6个盐桥。相比之下,对疾病类器官表型(fap1,2)的分析显示,新型盐桥的产生可能以显性-负性方式作用于全长APC,破坏APC功能并促进肿瘤发生。我们的研究强调了种系APC突变在结肠癌发生中的关键作用,揭示了特定突变如何影响疾病的严重程度。通过破译APC结构-功能关系,我们阐明了潜在的治疗方法和APC突变在临床表现之前的分子基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


