A new mononuclear hydrazone-based aluminum complex as a catalyst for solvent-free CO2 cycloaddition reaction
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Recently, due to increasing concerns over carbon dioxide emissions and their impact on the environment, the study and conversion of it into valuable products have been of great interest. In general, aluminum-based complexes can be a good choice for CO2 cycloaddition reactions due to their strong Lewis acidity. Hence, a mononuclear aluminum complex [Al(HL′)(H2O)3](H2O)2Cl containing hydrazonic based ligand (sodium 4-hydroxy-3-{[2-(pyridine-4‑carbonyl)hydrazinylidene]methyl}benzene-1-sulfonate) was synthesized and identified by FT-IR, 1H NMR, XRD and single-crystal X-ray crystallography. The ligand coordinated to aluminum in enolate-phenolate form through oxygen atoms of phenolate and enolate and nitrogen atoms of imine. Water molecules complete the distorted octahedral geometry around the metal center. The XRD pattern of the complex exhibited that it was stable up to around 120 °C. Therefore, the prepared compound was examined as a catalyst in the presence of quaternary ammonium (TBAB) as a co-catalyst for CO2 cycloaddition reaction under solvent-free conditions to synthesize cyclic carbonates via CO2 and epoxide. In this work, the reaction was performed under mild conditions. The conversion of around 80 % of epichlorohydrin was obtained using the catalyst and co-catalyst at 60 °C, 24 h, and 1 bar of CO2. A reusability study demonstrated that a catalyst can be used in at least three cycles without any decrease in catalytic activity under mild conditions.
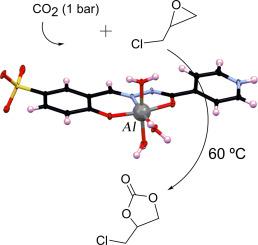
一种新型单核腙基铝配合物催化无溶剂CO2环加成反应
近年来,由于人们对二氧化碳排放及其对环境的影响越来越关注,研究并将其转化为有价值的产品已经引起了人们的极大兴趣。一般来说,铝基配合物由于具有较强的刘易斯酸度,可以成为CO2环加成反应的良好选择。因此,合成了含腙基配体(4-羟基-3-{[2-(吡啶-4 -羰基)肼基]甲基}苯-1磺酸钠)的单核铝配合物[Al(HL)(H2O)3](H2O)2Cl],并用FT-IR、1H NMR、XRD和单晶x射线晶体学对其进行了鉴定。该配体通过酚酸酯和烯酸酯的氧原子和亚胺的氮原子与铝形成烯酸酯-酚酸酯的配位。水分子围绕金属中心完成扭曲的八面体几何。该配合物的XRD谱图表明,该配合物在120°C左右是稳定的。因此,在季铵(TBAB)存在的情况下,将所制备的化合物作为催化剂,在无溶剂条件下,通过CO2和环氧化物合成环状碳酸盐,作为CO2环加成反应的助催化剂。在这项工作中,反应是在温和的条件下进行的。在60℃,24 h, 1 bar CO2条件下,催化剂和助催化剂的环氧氯丙烷转化率约为80%。一项可重复使用性研究表明,在温和的条件下,催化剂至少可以使用三个循环而不降低催化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


