Directing protonation equilibrium and reduction in [Fe2(CO)6{μ-(SCH2)2(Ph)P=O] via tailored acid modulation
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
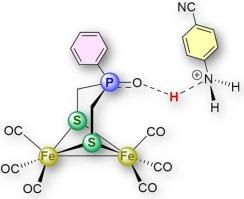
This study investigates how acid interactions at the phosphine oxide (P=O) site of the diiron dithiolato complex [Fe₂(CO)₆{(μ-SCH₂)₂(Ph)P=O}] (1) influence its reduction behavior. Using cyclic voltammetry and density functional theory (DFT) calculations, we show that the nature and strength of the acid dictate the interaction mode at the P![]() O site; either via hydrogen bonding or protonation. Pyridinium tetrafluoroborate ([HPy][BF₄], pKaMeCN = 12.53) induces hydrogen bonding, leading to a modest anodic shift (∼80 mV), whereas p-cyanoanilinium tetrafluoroborate ([HCA][BF₄], pKaMeCN = 7.00) facilitates protonation, causing a more pronounced shift (∼200 mV). These findings highlight the role of P
O site; either via hydrogen bonding or protonation. Pyridinium tetrafluoroborate ([HPy][BF₄], pKaMeCN = 12.53) induces hydrogen bonding, leading to a modest anodic shift (∼80 mV), whereas p-cyanoanilinium tetrafluoroborate ([HCA][BF₄], pKaMeCN = 7.00) facilitates protonation, causing a more pronounced shift (∼200 mV). These findings highlight the role of P![]() O in modulating the redox properties of complex 1 and provide insight into designing proton-responsive hydrogenase mimics.
O in modulating the redox properties of complex 1 and provide insight into designing proton-responsive hydrogenase mimics.
通过定制酸调制来指导[Fe2(CO)6{μ-(SCH2)2(Ph)P=O]的质子化平衡和还原
本文研究了二铁二硫腙配合物[Fe₂(CO)₆{(μ-SCH₂)₂(Ph)P=O}](1)在氧化膦(P=O)位点的酸相互作用对其还原行为的影响。利用循环伏安法和密度泛函理论(DFT)计算,我们发现酸的性质和强度决定了PO位点的相互作用模式;通过氢键或质子化。四氟硼酸吡啶([HPy][BF₄],pKaMeCN = 12.53)诱导氢键,导致适度的阳极移位(~ 80 mV),而对四氟硼酸氰胺([HCA][BF₄],pKaMeCN = 7.00)促进质子化,引起更明显的移位(~ 200 mV)。这些发现强调了PO在调节复合物1的氧化还原特性中的作用,并为设计质子响应的氢化酶模拟物提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


