N-acetylaspartate from fat cells regulates postprandial body temperature
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
N-acetylaspartate (NAA), the brain’s second most abundant metabolite, provides essential substrates for myelination through its hydrolysis1. However, the physiological roles of NAA in other tissues remain unknown. Here, we show that aspartoacylase (ASPA) expression in white adipose tissue (WAT) governs blood NAA levels for postprandial body temperature regulation. Genetic ablation of Aspa in mice resulted in systemically elevated NAA levels, and the ensuing accumulation in WAT stimulated pyrimidine production. Stable isotope tracing confirmed higher incorporation of glucose-derived carbon into pyrimidine metabolites in Aspa knockout cells. Additionally, serum NAA levels positively correlated with the abundance of the pyrimidine intermediate orotidine 5′-monophosphate, and this relationship predicted lower body mass index in humans. Using whole-body and tissue-specific knockout mouse models, we observed that fat cells provided plasma NAA and suppressed postprandial body temperature elevation. Moreover, unopposed NAA from adipocytes greatly enhanced whole-body glucose disposal exclusively in WAT. Exogenous NAA also increased plasma pyrimidines and lowered body temperature. These data place WAT-derived NAA as an endocrine regulator of postprandial body temperature and define broader roles for metabolic homeostasis. Aspartoacylase expression in white adipose tissue regulates circulating levels of N-acetylaspartate, which in turn modulates plasma pyrimidine levels and regulates postprandial body temperature.

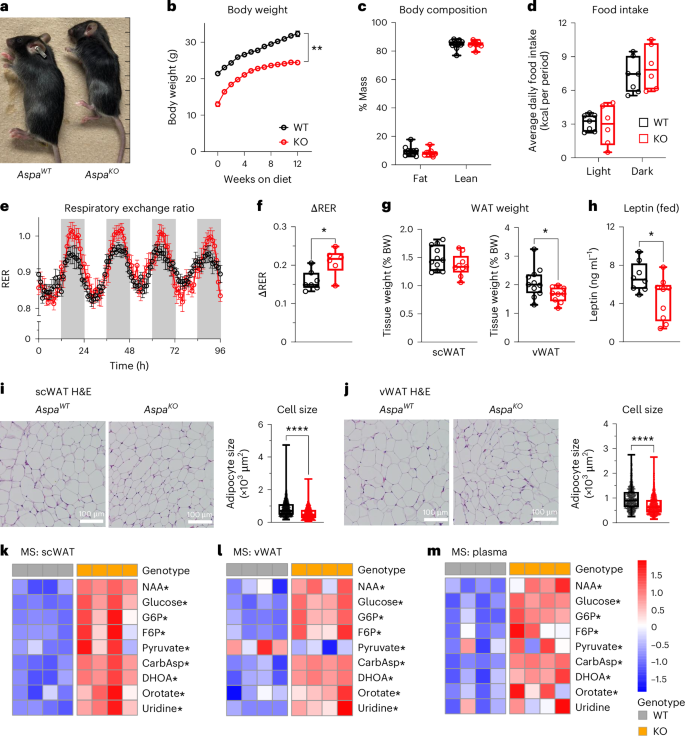
来自脂肪细胞的n -乙酰天冬氨酸调节餐后体温
n -乙酰天冬氨酸(NAA)是大脑中第二丰富的代谢物,通过水解为髓鞘形成提供必要的底物。然而,NAA在其他组织中的生理作用尚不清楚。在这里,我们发现白色脂肪组织(WAT)中的天冬氨酸酰化酶(ASPA)表达控制着餐后体温调节的血液NAA水平。小鼠Aspa基因消融导致NAA水平全身性升高,随后WAT的积累刺激了嘧啶的产生。稳定同位素示踪证实,在Aspa敲除细胞中,葡萄糖衍生的碳更多地掺入嘧啶代谢物中。此外,血清NAA水平与嘧啶中间体orotidine 5 ' - monophospate的丰度呈正相关,这种关系预示着人类较低的体重指数。使用全身和组织特异性敲除小鼠模型,我们观察到脂肪细胞提供血浆NAA并抑制餐后体温升高。此外,来自脂肪细胞的非对抗NAA极大地增强了WAT的全身葡萄糖处理。外源性NAA也增加血浆嘧啶和降低体温。这些数据表明,wat衍生的NAA是餐后体温的内分泌调节剂,并在代谢稳态中发挥更广泛的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


