Heat stress targets and degrades BCR::ABL1 oncoproteins to overcome drug-resistance in Philadelphia chromosome-positive acute lymphoblastic leukemia
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
BCR::ABL1 oncofusion protein drives Philadelphia-chromosome positive acute lymphoblastic leukemia (Ph+ ALL), making it a critical therapeutic target. Tyrosine kinase inhibitors (TKIs) targeting BCR::ABL1 have revolutionized the treatment of Ph+ ALL patients. However, mutations in the kinase domain of BCR::ABL1 commonly impair the sensitivity to TKIs, resulting in drug resistance and poor prognosis in Ph+ ALL. Here we report that heat stress selectively destabilizes BCR::ABL1 and its common drug-resistant mutants without affecting the native BCR and ABL proteins through inducing liquid-to-solid phase transition. Mechanistic studies revealed that heat stress facilitated recruitment of BCR::ABL1 signaling components (e.g., SHIP2, Sts1, PI3K-p85α and Shc) in a kinase activity dependent manner and stimulated BCR::ABL1 oligomerization through its coiled-coil domain, resulting in formation of a large, thermally unstable signaling complex. This process triggers non-canonical K27-linked ubiquitination mediated by c-Cbl E3 ubiquitin ligase, ultimately leading to BCR::ABL1 degradation via the ubiquitin-proteasome pathway. Functionally, heat stress effectively suppressed proliferation of BCR::ABL1-driven leukemia cells, including drug resistant mutants in vitro and decreased tumor burden in vivo. Our findings established that thermal-based therapy as a novel strategy to selectively target and degrade both unmutated and drug-resistant BCR::ABL1 oncoproteins, offering a promising adjuvant approach to overcome TKI resistance in Ph+ ALL.

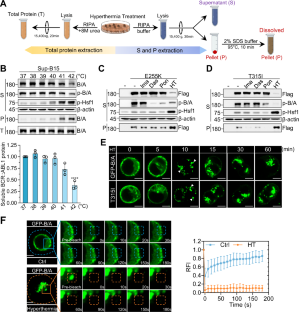
热应激靶向并降解BCR::ABL1癌蛋白以克服费城染色体阳性急性淋巴细胞白血病的耐药
BCR::ABL1混淆蛋白驱动philadelphia -染色体阳性急性淋巴细胞白血病(Ph+ ALL),使其成为关键的治疗靶点。靶向BCR::ABL1的酪氨酸激酶抑制剂(TKIs)已经彻底改变了Ph+ ALL患者的治疗。然而,在Ph+ ALL中,BCR::ABL1激酶结构域的突变通常会损害TKIs的敏感性,导致耐药和预后不良。在这里,我们报道了热应激选择性地破坏BCR::ABL1及其常见的耐药突变体的稳定性,而不影响天然BCR和ABL蛋白,通过诱导液相到固相转变。机制研究表明,热应激促进BCR::ABL1信号成分(如SHIP2、Sts1、PI3K-p85α和Shc)以激酶活性依赖的方式募集,并通过其线圈结构域刺激BCR::ABL1寡聚化,导致形成一个大的、热不稳定的信号复合物。这一过程触发了由c-Cbl E3泛素连接酶介导的非规范k27连锁泛素化,最终通过泛素-蛋白酶体途径导致BCR::ABL1降解。在功能上,热应激在体外有效抑制BCR:: abl1驱动的白血病细胞(包括耐药突变体)的增殖,并在体内降低肿瘤负荷。我们的研究结果表明,热疗法是一种选择性靶向和降解未突变和耐药BCR::ABL1癌蛋白的新策略,为克服Ph+ ALL中TKI耐药提供了一种有希望的辅助方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


