Investigation of the solubility of naphthalene Based nitro-derivatives in different strengths of sulfuric acid and temperatures
IF 2.7
Q2 Chemistry
引用次数: 0
Abstract
The solubilities of 1,5-dinitronaphthalene (1,5-DNN), 1,8-dinitronaphthalene (1,8-DNN), and 1-nitronaphthalene (NN) in different strengths of sulfuric acid ( % w/w) were determined using isothermal saturation method. Measurements were conducted at six temperatures ranging from 308.15 K to 333.15 K. The solubility was found to increase with temperature for all the compounds. The relationship between solubility and acid strength exhibited anomalous behavior with nitro derivatives exhibiting a steep rise in solubility at higher strengths of acid. The experimental solubility data was correlated using modified Apelblat, λ-h, van't Hoff and NRTL models. Good agreement was observed between the model predictions and the experimental data, with the modified Apelblat equation giving the best fit. Mixing thermodynamic properties were calculated based on the NRTL model. The results indicate that the dissolution process for all the three compounds is spontaneous, endothermic, and entropy-driven across the range of acid strengths investigated.
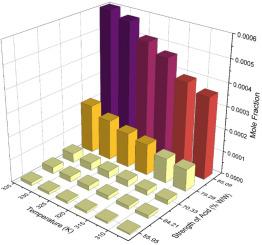
萘基硝基衍生物在不同强度硫酸和温度下的溶解度研究
采用等温饱和法测定了1,5-二硝基萘(1,5- dnn)、1,8-二硝基萘(1,8- dnn)和1-硝基萘(NN)在不同浓度硫酸(% w/w)中的溶解度。测量在308.15 K至333.15 K的六个温度范围内进行。发现所有化合物的溶解度随温度升高而增加。溶解度与酸强度之间的关系表现出异常行为,硝基衍生物在较高的酸强度下溶解度急剧上升。采用改进的Apelblat、λ-h、van't Hoff和NRTL模型对实验溶解度数据进行关联。模型预测结果与实验数据吻合较好,修正后的Apelblat方程拟合效果最好。基于NRTL模型计算了混合热力学性质。结果表明,在酸强度范围内,这三种化合物的溶解过程都是自发的、吸热的和熵驱动的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Data Collections
Chemistry-Chemistry (all)
CiteScore
6.10
自引率
0.00%
发文量
169
审稿时长
24 days
期刊介绍:
Chemical Data Collections (CDC) provides a publication outlet for the increasing need to make research material and data easy to share and re-use. Publication of research data with CDC will allow scientists to: -Make their data easy to find and access -Benefit from the fast publication process -Contribute to proper data citation and attribution -Publish their intermediate and null/negative results -Receive recognition for the work that does not fit traditional article format. The research data will be published as ''data articles'' that support fast and easy submission and quick peer-review processes. Data articles introduced by CDC are short self-contained publications about research materials and data. They must provide the scientific context of the described work and contain the following elements: a title, list of authors (plus affiliations), abstract, keywords, graphical abstract, metadata table, main text and at least three references. The journal welcomes submissions focusing on (but not limited to) the following categories of research output: spectral data, syntheses, crystallographic data, computational simulations, molecular dynamics and models, physicochemical data, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


