Modulation of electronic structure of metal-free graphdiyne via precise nitrogen modification for oxygen evolution reaction
引用次数: 0
Abstract
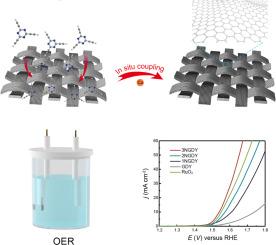
The oxygen evolution reaction (OER) is essential for energy conversion and storage but is hindered by sluggish kinetics, low efficiency, and high overpotentials. Although RuO₂ and IrO₂ are efficient catalysts, their high cost and scarcity limit their large-scale application. In contrast, nonmetallic catalysts have gained traction as promising alternatives due to their cost-effectiveness, high stability, and environmental sustainability. The OER efficiency depends on optimal adsorption/desorption of oxygen intermediates, such as *O, OH*, and OOH*, on the catalyst surface. The electronic structure of carbon materials can be optimized via nitrogen doping, which introduces a higher polarity than carbon atoms, thereby optimizing the adsorption free energy of oxygen species during an OER. However, conventional high-temperature pyrolysis methods suffer from limitations such as inaccuracy and high energy consumption. The unique and facile bottom-up synthesis of graphdiyne (GDY) enables precise control over the doping positions of the three sp²-N atoms in GDY (1NGDY, 2NGDY, and 3NGDY) via monomer design engineering. By integrating density functional theory (DFT) calculations with experimental validation, we tailored the adsorption free energy of the oxygen intermediates in the OER, thereby optimizing the rate-determining step of *OOH generation. Among these three kinds of nitrogen-doped GDY catalysts, 3NGDY which incorporates three sp2-N atoms exhibited the optimal electrocatalytic performance, achieving a current density of 10 mA cm⁻² in 1 M KOH with a low overpotential of approximately 310 mV. This study demonstrates the significant potential of GDY-based metal-free catalysts in the development of cost-effective, high-performance electrocatalysts.
无金属石墨炔在析氧反应中精确氮修饰的电子结构调制
析氧反应(OER)是能量转化和储存的必要条件,但由于动力学缓慢、效率低和过电位高而受到阻碍。虽然RuO₂和IrO₂是高效的催化剂,但它们的高成本和稀缺性限制了它们的大规模应用。相比之下,非金属催化剂由于其成本效益、高稳定性和环境可持续性而成为有希望的替代品。OER效率取决于氧中间体(如*O, OH*和OOH*)在催化剂表面的最佳吸附/解吸。通过氮掺杂可以优化碳材料的电子结构,引入比碳原子更高的极性,从而优化氧在OER过程中的吸附自由能。然而,传统的高温热解方法存在精度不高、能耗高等局限性。石墨炔(GDY)独特而简便的自下而上合成方法可以通过单体设计工程精确控制GDY中三个sp²-N原子(1NGDY, 2NGDY和3NGDY)的掺杂位置。通过将密度泛函理论(DFT)计算与实验验证相结合,我们定制了OER中氧中间体的吸附自由能,从而优化了*OOH生成的速率决定步骤。在三种氮掺杂GDY催化剂中,含有3个sp2-N原子的3NGDY表现出最佳的电催化性能,在1 M KOH中电流密度为10 mA cm⁻²,过电位约为310 mV。这项研究表明,基于gdd的无金属催化剂在开发经济高效的电催化剂方面具有巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


