Neutrophil-mediated alteration of CXCL1/IL-8/AR signaling promotes prostate cancer cell proliferation
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
The role of immune cells, particularly neutrophils, in the prostate cancer (PCa) progression remains poorly understood. In this study, we investigated the impact of neutrophils on PCa progression using an in vitro co-culture migration assay. Our findings revealed that PCa cells recruited more neutrophils than normal prostate epithelial cells. Importantly, the recruitment of neutrophils to PCa cells led to increased PCa cell proliferation. Further mechanistic investigations revealed that co-culture of PCa cells with neutrophils led to increased secretion of the chemokine CXCL1. This, in turn, stimulated neutrophils to produce the cytokine IL-8. The enhanced CXCL1/IL-8 signaling axis subsequently amplified androgen receptor (AR) signaling in PCa cells, thereby promoting their proliferation. Disruption of this pathway via IL-8 neutralizing antibodies or AR knockdown reversed the neutrophil-induced PCa cell proliferation. These findings were validated in a mouse model and further supported by clinical sample analysis. Collectively, our study highlights the therapeutic potential of targeting the newly identified signaling cascade involving infiltrating neutrophils within the PCa microenvironment. Understanding and modulating this pathway may offer novel strategies to suppress PCa progression.
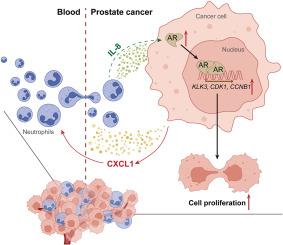
中性粒细胞介导的CXCL1/IL-8/AR信号的改变促进前列腺癌细胞的增殖
免疫细胞,特别是中性粒细胞在前列腺癌(PCa)进展中的作用仍然知之甚少。在这项研究中,我们通过体外共培养迁移试验研究了中性粒细胞对前列腺癌进展的影响。我们的研究结果显示,前列腺癌细胞比正常前列腺上皮细胞募集了更多的中性粒细胞。重要的是,中性粒细胞向PCa细胞募集导致PCa细胞增殖增加。进一步的机制研究表明,PCa细胞与中性粒细胞共培养导致趋化因子CXCL1的分泌增加。这反过来刺激中性粒细胞产生细胞因子IL-8。增强的CXCL1/IL-8信号轴随后放大了PCa细胞中的雄激素受体(AR)信号,从而促进其增殖。通过IL-8中和抗体或AR敲低破坏这一途径可逆转中性粒细胞诱导的PCa细胞增殖。这些发现在小鼠模型中得到了验证,并得到了临床样本分析的进一步支持。总的来说,我们的研究强调了针对PCa微环境中浸润中性粒细胞的新发现的信号级联的治疗潜力。理解和调节这一途径可能提供抑制前列腺癌进展的新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


