Unveiling the role of TAM-derived extracellular vesicles in glioma progression through Treg polarization and immune suppression
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
In the context of gliomas, tumor-associated macrophages (TAMs) and regulatory T cells (Tregs) play crucial roles in shaping the tumor microenvironment (TME). This study focused on elucidating the mechanism by which TAM-derived extracellular vesicles (EVs) influence Treg differentiation and contribute to glioma progression. Through comprehensive single-cell RNA sequencing (scRNA-seq) analysis, the glioma TME was characterized by an abundance of TAMs exhibiting M2 polarization and increased Treg differentiation. Notably, TAM EVs were identified as potent inducers of Treg differentiation, with the downregulation of Bactericidal/Permeability-Increasing protein (BPI) being associated with this process. In vivo experiments utilizing a mouse model of glioma further demonstrated that TAM-derived EVs promoted glioma growth by enhancing Treg-mediated immunosuppression while dampening pro-inflammatory responses. This study highlights the critical role of TAM-derived EVs in modulating Treg differentiation and supporting glioma progression, suggesting that interventions targeting TAM EVs or regulating BPI expression could offer novel therapeutic avenues for combating immune suppression and inhibiting glioma development.
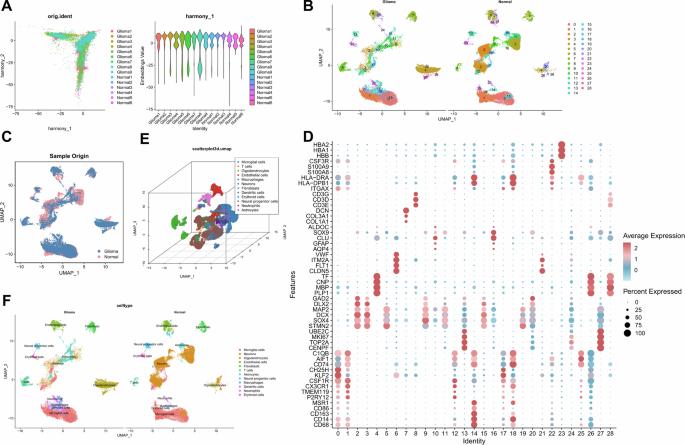
揭示tam衍生的细胞外囊泡通过Treg极化和免疫抑制在胶质瘤进展中的作用。
在胶质瘤的背景下,肿瘤相关巨噬细胞(tam)和调节性T细胞(Tregs)在形成肿瘤微环境(TME)中起着至关重要的作用。本研究的重点是阐明tam衍生的细胞外囊泡(EVs)影响Treg分化并促进胶质瘤进展的机制。通过全面的单细胞RNA测序(scRNA-seq)分析,胶质瘤TME的特征是大量的tam表现出M2极化和Treg分化增加。值得注意的是,TAM ev被鉴定为Treg分化的有效诱导剂,其杀菌/通透性增加蛋白(BPI)的下调与这一过程有关。利用小鼠胶质瘤模型的体内实验进一步证明,tam衍生的ev通过增强treg介导的免疫抑制,同时抑制促炎反应,促进胶质瘤的生长。这项研究强调了TAM衍生的ev在调节Treg分化和支持胶质瘤进展中的关键作用,表明针对TAM ev或调节BPI表达的干预可能为对抗免疫抑制和抑制胶质瘤的发展提供新的治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


