The development but not the maintenance of phrenic and sympathetic long-term facilitation after acute intermittent hypoxia requires nucleus tractus solitarii H2O2
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Acute exposure to intermittent hypoxia (AIH) produces prolonged increases (long-term facilitation, LTF) in phrenic (PhrNA) and sympathetic (SNA) nerve activity (pLTF and sLTF, respectively) during non-hypoxic periods, and augments cardiorespiratory responses to hypoxia. We recently showed that neuronal activity in the nucleus tractus solitarii (nTS) is required for the induction and maintenance of LTF. However, the specific mechanisms involved were not determined. Because bouts of deoxygenation/reoxygenation produce reactive oxygen species and H2O2 contributes to plasticity in the nTS, we hypothesized that nTS H2O2 contributes to AIH-induced LTF and augmented hypoxic responses. We reduced H2O2 within the nTS acutely by nanoinjecting catalase or chronically by overexpressing catalase via an adenovirus vector. We then evaluated PhrNA and splanchnic SNA (SSNA) in animals subjected to AIH or time control. In control rats subjected to nTS nanoinjections of aCSF or overexpression of eGFP, AIH produced pLTF and sLTF, and augmented PhrNA responses to hypoxia. Reducing nTS H2O2 by either nTS nanoinjections or overexpression of catalase markedly attenuated the development of pLTF and sLTF. Augmented hypoxic responses due to AIH also were diminished. In contrast, after LTF had developed, nanoinjection of catalase had no effect on the magnitude of either PhrNA or SSNA although inhibiting nTS neuronal activity after LTF development reduced pLTF. The data indicate that nTS H2O2 is required for AIH-induced pLTF and sLTF, as well as augmentation of responses to hypoxia. Moreover, while nTS neuronal activity is essential to the maintenance of pLTF once developed, ongoing increases in H2O2 are not required.
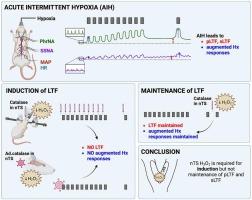
急性间断性缺氧后膈神经和交感神经的长期促进作用需要孤立束核的H2O2。
急性暴露于间歇性缺氧(AIH)会在非缺氧期间延长膈(PhrNA)和交感(SNA)神经活动(分别为pLTF和sLTF)的增加(长期促进,LTF),并增强对缺氧的心肺反应。我们最近发现孤立束核(nTS)的神经元活动是LTF的诱导和维持所必需的。但是,所涉及的具体机制尚未确定。由于多次脱氧/再氧化产生活性氧,H2O2有助于nTS的可塑性,我们假设nTS H2O2有助于aih诱导的LTF和增强的缺氧反应。我们通过纳米注射过氧化氢酶或通过腺病毒载体过表达过氧化氢酶来减少nTS内的H2O2。然后,我们评估了AIH或时间控制动物的PhrNA和内脏SNA (SSNA)。在nTS纳米注射aCSF或过表达eGFP的对照大鼠中,AIH产生pLTF和sLTF,并增强PhrNA对缺氧的反应。通过nTS纳米注射剂或过氧化氢酶的过表达来减少nTS H2O2,可显著减弱pLTF和sLTF的发展。AIH引起的增强缺氧反应也减弱了。相比之下,LTF发生后,纳米注射过氧化氢酶对PhrNA或SSNA的大小没有影响,尽管LTF发生后抑制nTS神经元活性降低了pLTF。数据表明,nTS H2O2是aih诱导的pLTF和sLTF以及对缺氧反应增强所必需的。此外,nTS神经元的活动对于pLTF的维持至关重要,但H2O2的持续增加并不需要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


