LncRNA SChLAP1 Promotes Cancer Cell Proliferation and Invasion Via Its Distinct Structural Domains and Conserved Regions
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Long non-coding RNAs (lncRNAs) play key roles in a range of biological processes and disease progression. Despite their functional significance and therapeutic potential, lncRNAs’ mechanisms of action remain understudied. One such lncRNA is the Second Chromosome Locus Associated with Prostate-1 (SChLAP1). SChLAP1 is overexpressed in malignant prostate cancer and is associated with unfavorable patient outcomes, such as metastasis and increased mortality. In this study, we demonstrated that SChLAP1 possesses distinct structural domains and conserved regions that may contribute to its function. We determined the secondary structure of SChLAP1 using chemical probing methods combined with mutational profiling (DMS-MaP and SHAPE-MaP). Our in vitro secondary structural model revealed that SChLAP1 consists of two distinct secondary structural modules located at its 5′ and 3′ ends, both featuring regions with a high degree of structural organization. Our in vivo chemical probing identified structurally stable regions and areas that may undergo specific structural rearrangements in the cellular context. Overexpression of the modules led to a notable increase in cancer cell proliferation and invasion, proving their functional significance in the oncogenicity of SChLAP1. In conclusion, we discovered functionally important, independent modules with well-defined structures of SChLAP1. These results will serve as a guide to explore the detailed molecular mechanisms by which SChLAP1 promotes aggressive prostate cancer, ultimately contributing to the development of SChLAP1 as a novel therapeutic target.
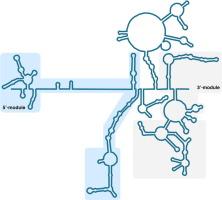
LncRNA SChLAP1通过其独特的结构域和保守区域促进癌细胞的增殖和侵袭。
长链非编码rna (lncRNAs)在一系列生物过程和疾病进展中发挥关键作用。尽管lncrna具有重要的功能和治疗潜力,但其作用机制仍未得到充分研究。其中一个lncRNA是与前列腺-1相关的第二染色体位点(SChLAP1)。SChLAP1在恶性前列腺癌中过表达,并与不良的患者预后相关,如转移和死亡率增加。在这项研究中,我们证明了SChLAP1具有独特的结构域和可能有助于其功能的保守区域。我们利用化学探针方法结合突变谱(DMS-MaP和SHAPE-MaP)确定了SChLAP1的二级结构。我们的体外二级结构模型显示,SChLAP1由位于其5‘和3’端两个不同的二级结构模块组成,都具有高度结构组织化的区域。我们的体内化学探测确定了结构稳定的区域和可能在细胞环境中进行特定结构重排的区域。这些模块的过表达导致癌细胞增殖和侵袭显著增加,证明了它们在SChLAP1致癌性中的功能意义。总之,我们发现了功能重要、结构明确的SChLAP1独立模块。这些结果将为探索SChLAP1促进侵袭性前列腺癌的详细分子机制提供指导,最终促进SChLAP1作为一种新的治疗靶点的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


