Icariin modulates the tumor microenvironment in colorectal cancer by targeting M2 macrophage polarization via PI3K/AKT pathway
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Colorectal cancer (CRC) progression is influenced by intricate interactions in the tumor microenvironment (TME), with M2-polarized macrophages being key players in enhancing tumor growth and suppressing the immune response. Icariin (ICA), a bioactive flavonoid isolated from Epimedium brevicornu Maxim., exhibits potential antitumor properties. Nevertheless, its role in modulating macrophage-mediated immunosuppression in CRC remains unclear. To assess ICA's effects on CRC malignancy within an M2-enriched microenvironment, we established a CRC cell/M2 macrophage co-culture system. ICA suppressed CRC cell proliferation, migration, and invasion in co-culture with M2 macrophages. M2 polarization markers and PI3K/AKT pathway modulation were analyzed by western blot and qRT-PCR. Mechanistic studies revealed that ICA inhibited M2 polarization and suppressed the phosphorylation of PI3K and AKT. In vivo validation utilized two models, an AOM/DSS-induced CRC model and a syngeneic CT26-WT implantation system, evaluating both tumor progression and macrophage phenotype alterations. We found that ICA attenuated tumor growth and reduced M2 macrophage infiltration. Collectively, these finding demonstrated that ICA suppressed M2 macrophage polarization via PI3K/AKT signaling pathway, thereby inhibiting CRC progression. This study reveals a previously unrecognized mechanism by which ICA inhibits CRC and demonstrates its potential as a promising therapeutic agent for CRC treatment.
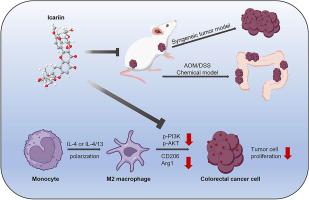
淫羊藿苷通过PI3K/AKT通路靶向M2巨噬细胞极化调节结直肠癌肿瘤微环境
结直肠癌(CRC)的进展受到肿瘤微环境(TME)中复杂的相互作用的影响,其中m2极化巨噬细胞是促进肿瘤生长和抑制免疫反应的关键参与者。淫羊藿苷(ICA)是从淫羊藿中分离得到的一种具有生物活性的类黄酮。显示出潜在的抗肿瘤特性。然而,其在CRC中调节巨噬细胞介导的免疫抑制中的作用尚不清楚。为了评估ICA在富含M2的微环境中对CRC恶性肿瘤的影响,我们建立了CRC细胞/M2巨噬细胞共培养系统。在与M2巨噬细胞共培养时,ICA抑制结直肠癌细胞的增殖、迁移和侵袭。采用western blot和qRT-PCR分析M2极化标记和PI3K/AKT通路调节。机制研究表明,ICA抑制M2极化,抑制PI3K和AKT的磷酸化。体内验证采用两种模型,AOM/ dss诱导的CRC模型和同基因CT26-WT植入系统,评估肿瘤进展和巨噬细胞表型改变。我们发现ICA能抑制肿瘤生长,减少M2巨噬细胞浸润。综上所述,这些发现表明ICA通过PI3K/AKT信号通路抑制M2巨噬细胞极化,从而抑制CRC的进展。本研究揭示了ICA抑制结直肠癌的一种以前未被认识的机制,并证明了其作为结直肠癌治疗的一种有前景的治疗剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


