Decoding MHC loss: Molecular mechanisms and implications for immune resistance in cancer
Abstract
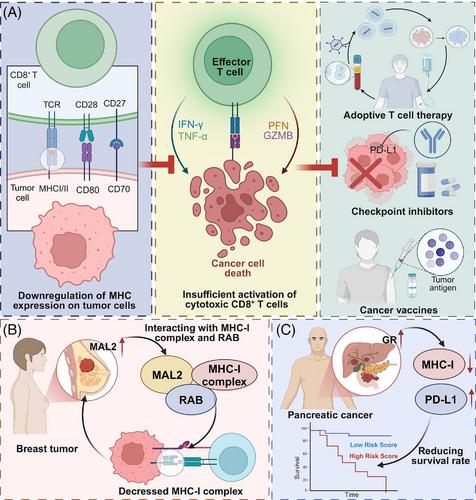
Loss or downregulation of major histocompatibility complex (MHC) molecules represents a key mechanism by which tumours escape immune recognition and acquire resistance to immunotherapeutic interventions. This review focuses on the central regulatory pathways. These includes transcriptional repression, lysosomal degradation, and post-translational modifications that disrupt MHC stability, trafficking, and surface expression. We highlight how these mechanisms impair antigen presentation and contribute to tumour immune evasion. In addition, we explore emerging therapeutic strategies focused on reactivating MHC expression to enhance tumour immunogenicity and improve the efficacy of immunotherapy. Finally, we discuss the translational potential of these approaches and the remaining challenges, including tumour heterogeneity, immunotoxicity and dynamic regulation within the tumour microenvironment, that must be addressed to optimize MHC-targeted interventions in cancer immunotherapy.
Highlights
-
Tumour cells evade immune surveillance by downregulating MHC expression through transcriptional repression, lysosomal degradation and post-translational modifications.
-
Pharmacological agents interventing epigenetic and metabolic can upregulate MHC expression and improve T cell activation.
-
Combination strategies potentiate immunotherapy efficacy by reinvigorating tumour immunogenicity.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


