Integrated single-cell and spatial transcriptomics uncover distinct cellular subtypes involved in neural invasion in pancreatic cancer
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Nerves are integral to tumor biology, yet the peri- and intra-neural microenvironment and their roles in cancer-neural invasion (NI) remain underexplored. Here, we perform single-cell/single-nucleus RNA sequencing (sc/snRNA-seq) and spatial transcriptomics on 62 samples from 25 pancreatic ductal adenocarcinoma (PDAC) patients, mapping cellular composition, lineage dynamics, and spatial organization across varying NI statuses. Tertiary lymphoid structures are abundant in low-NI tumor tissues and co-localize with non-invaded nerves, while NLRP3+ macrophages and cancer-associated myofibroblasts surround invaded nerves in high-NI tissues. We identify a unique endoneurial NRP2+ fibroblast population and characterize three distinct Schwann cell subsets. TGFBI+ Schwann cells locate at the leading edge of NI, can be induced by transforming growth factor β (TGF-β) signaling, promote tumor cell migration, and correlate with poor survival. We also identify basal-like and neural-reactive malignant subpopulations with distinct morphologies and heightened NI potential. This landscape depicting tumor-associated nerves highlights critical cancer-immune-neural interactions in situ and enlightens treatment development targeting NI.
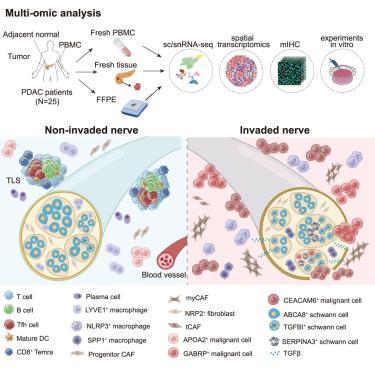
整合的单细胞和空间转录组学揭示了参与胰腺癌神经侵袭的不同细胞亚型
神经是肿瘤生物学中不可或缺的一部分,但神经周围和神经内微环境及其在肿瘤-神经侵袭(NI)中的作用仍未得到充分研究。在这里,我们对来自25名胰腺导管腺癌(PDAC)患者的62个样本进行了单细胞/单核RNA测序(sc/snRNA-seq)和空间转录组学,绘制了不同NI状态下的细胞组成、谱系动力学和空间组织。三级淋巴样结构在低ni肿瘤组织中丰富,与未浸润神经共定位,而在高ni肿瘤组织中,NLRP3+巨噬细胞和癌相关肌成纤维细胞围绕浸润神经。我们鉴定了一种独特的神经内膜NRP2+成纤维细胞群,并表征了三种不同的雪旺细胞亚群。TGFBI+雪旺细胞位于NI的前沿,可被转化生长因子β (TGF-β)信号诱导,促进肿瘤细胞迁移,与生存不良相关。我们也发现基底样和神经反应性恶性亚群具有不同的形态和高NI电位。这幅描绘肿瘤相关神经的图景强调了关键的原位癌症免疫-神经相互作用,并启发了针对NI的治疗开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


